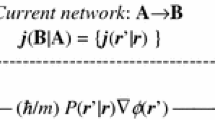Abstract
The information-theoretic (IT) approach to the electronic structure of molecules, called the Communication Theory of the chemical bond, is used to explore the entropy/information “bond-orders” of the system Valence-Bond (VB) structures, and their covalent/ionic composition. The molecular communication channel in atomic resolution is interpreted as the ensemble-average of the elementary information networks corresponding to the system elementary VB structures, with the probability-weights generated by the coefficients of the familiar VB expansion of the system wave-function. The elementary probability-scattering networks corresponding to a single ionic and covalent bonds of the system constituent diatomic fragments, to be used to construct the communication systems of any VB structure, are identified and their entropy/information descriptors are summarized. The overall bond multiplicities of VB structures are generated using the relevant grouping rules for the entropy/information quantities and the molecular IT bond-order and its covalent/ionic components are obtained as the ensemble averages of the corresponding descriptors of individual VB structures. Illustrative applications to the hydrogen molecule and π-bonds in allyl, butadiene and benzene are reported and discussed. Equating the VB-ensemble average quantities for these π-electron systems with those resulting from the Hückel Molecular Orbital (MO) theory then allows one to extract the VB relative covalency and ionicity measures for all chemical bonds in the molecule. The simple MO and VB descriptions of the hydrogen molecule are compared and the role of a strong overlap between the VB structures is investigated in a more detail. Finally, the entropy/information descriptors of the information distribution in the resultant promolecule → Atoms-in-H2 channel resulting from the sequential information cascade consisting of the promolecular and VB probability propagation stages is examined to determine the dependence of the flow of information on the adopted reference system.
Similar content being viewed by others
References
R.A. Fisher, Proc. Cambridge Phil. Soc. 22, 700 (1925); see also: B.R. Frieden, Physics from the Fisher Information—a Unification (Cambridge University Press, Cambridge, 2000)
C.E. Shannon, Bell System Tech. J. 27, 379, 623 (1948); see also: C.E. Shannon, W. Weaver, The Mathematical Theory of Communication (University of Illinois, Urbana, 1949)
S. Kullback, R.A. Leibler, Ann. Math. Stat. 22, 79 (1951); see also: S. Kullback, Information Theory and Statistics (Wiley, New York, 1959)
Abramson N.: Information Theory and Coding. McGraw-Hill, New York (1963)
R.F. Nalewajski, Information Theory of Molecular Systems (Elsevier, Amsterdam, 2006) and refs. therein
Nalewajski R.F.: J. Phys. Chem. A 104, 11940 (2000)
R.F. Nalewajski, Mol. Phys. 102, 531, 547 (2004)
Nalewajski R.F.: Mol. Phys. 103, 451 (2005)
R.F. Nalewajski, Mol. Phys. 104, 493, 1977, 2533 (2006)
Nalewajski R.F.: Mol. Phys. 104, 365 (2006)
Nalewajski R.F.: Struct. Chem. 15, 391 (2004)
Nalewajski R.F.: J. Math. Chem. 38, 43 (2005)
Nalewajski R.F.: Theoret. Chem. Acc. 114, 4 (2005)
R.F. Nalewajski, K. Jug, in Reviews of Modern Quantum Chemistry: A Celebration of the Contributions of Robert G. Parr, vol. I, ed. by K.D. Sen (World Scientific, Singapore, 2002), p. 148
Nalewajski R.F.: Chem. Phys. Lett. 386, 265 (2004)
R.F. Nalewajski, J. Math. Chem. (in press), doi:10.1007/s10910-007-9345-4
R.F. Nalewajski, Mol. Phys., 104, 2533, 3339 (2006)
Nalewajski R.F.: J. Phys. Chem. A 111, 4855 (2007)
Nalewajski R.F.: J. Math. Chem. 43, 265 (2006)
Nalewajski R.F.: J. Math. Chem. 43, 780 (2008)
R.F. Nalewajski, J. Math. Chem. (in press), doi:10.1007/s10910-007-9318-7
W. Heitler, F. London, Z. Physik 44, 455 (1927); for an English translation see: H. Hettema, Quantum Chemistry Classic Scientific Paper (World Scientific, Singapore, 2000); F. London, Z. Phys. 455, 46 (1928)
S. Shaik, in New Theoretical Concepts for Understanding Organic Reactions, NATO ASI Series, vol. C267, ed. by J. Bertran, I. G. Czismadia (Kluwer Academic Publ., Dordrecht, 1989), p. 165; S. Shaik, P.C. Hiberty, Adv. Quant. Chem. 26, 100 (1995)
Goddard W.A. III, Harding L.B.: Annu. Rev. Phys. Chem. 29, 363 (1978)
Murrell J.N., Carter S., Farantos S.C., Huxley P., Varandas A.J.C.: Molecular Potential Energy Functions. Wiley, New York (1984)
Eyring H., Walter J., Kimball G.E.: Quantum Chemistry. Wiley, New York (1958)
Barriol J.: Elements of Quantum Mechanics with Chemical Applications. Barnes & Noble, New York (1971)
O.K. Dawtjan, Quantum Chemistry (Higher School, Moscow, 1962), in Russian
G. Rumer, Göttingen Nachr. 377 (1932)
Jug K., Köster A.M.: J. Am. Chem. Soc. 112, 6772 (1990)
Berry R.S., Rice S.A., Ross J.: Physical Chemistry. Wiley, New York (1980)
Nalewajski R.F., Köster A.M., Jug K.: Theoret. Chim. Acta (Berl.) 85, 463 (1993)
R.F. Nalewajski, J. Mrozek, Int. J. Quantum Chem. 51, 187 (1994); R.F. Nalewajski, S.J. Formosinho, A.J.C. Varandas, J. Mrozek, Int. J. Quantum Chem. 52, 1153 (1994)
Nalewajski R.F., Mrozek J., Mazur G.: Can. J. Chem. 100, 1121 (1996)
Nalewajski R.F., Mrozek J., Michalak A.: Int. J. Quantum Chem. 61, 589 (1997)
Mrozek J., Nalewajski R.F., Michalak A.: Polish J. Chem. 72, 1779 (1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nalewajski, R.F. Communication-theory perspective on valence-bond theory. J Math Chem 45, 709–724 (2009). https://doi.org/10.1007/s10910-008-9385-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-008-9385-4