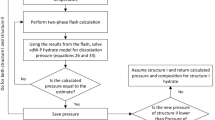
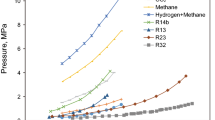
Abstract
This work presents a review of literature data of methane and carbon dioxide hydrate equilibrium at low temperatures. Constants of Arrhenius-type equation accurately determined for the mentioned lines which allow calculating the hydrate equilibrium pressure at any temperature below the quadruple point for both systems contain ice or supercooled water. Through intersection analysis, new accurate quadruple points were determined. Interpretations based on flash calculations by high accurate equations of states shown enthalpies of clathrate formation/dissociation, for equilibrium below quadruple point, lead to the similarity of Clapeyron and Clausius–Clapeyron approaches. Based on equality of equilibrium conditions at the quadruple point, new hydration numbers were calculated. Gamma–phi approach through high accurate equations of states of GERG-2008 and CG for the prediction of VHIw three-phase equilibrium line was evaluated. Commercial packages of Multiflash and PVTsim and open-source codes of CSMGem and CSMHYD were used to model the phenomena.











Similar content being viewed by others
References
E.D. Sloan Jr., C. Koh, Clathrate Hydrates of Natural Gases (CRC Press, Boca Raton, 2007)
U. Hiroki, H. Akiba, S. Akatsu, R. Ohmura, New J. Chem. 39, 8254–8262 (2015)
J.M. Brooks, H.B. Cox, W.R. Bryant, M.C. Kennicutt, R.G. Mann, T.J. McDonald, Org. Geochem. 10, 221–234 (1986)
M. Kastner, K.A. Kvenvolden, T.D. Lorenson, Earth Planet. Sci. Lett. 156, 173–183 (1998)
O. Mousis, J.L. Lunine, K.E. Mandt, E. Schindhelm, H.A. Weaver, S.A. Stern, J.H. Waite, R. Gladstone, A. Moudens, Icarus 225, 856–861 (2013)
D. Ambuehl, M.E. Madden, Icarus 234, 45–52 (2014)
R. Llopis, E. Torrella, R. Cabello, D. Sanchez, Int. J. Refrig. 35, 810–816 (2012)
A. McCulloch, J. Fluor. Chem. 123, 21–29 (2003)
W. Wu, B. Wong, W. Shi, X. Li, Renew. Sustain. Energy Rev. 31, 681–707 (2014)
J. Javanmardi, Kh Nasrifar, S.H. Najibi, M. Moshfeghian, Appl. Therm. Eng. 25, 1708–1723 (2005)
Z.R. Chong, S.H.B. Yang, P. Babu, P. Linga, X.S. Li, Appl. Energy 162, 1633–1652 (2016)
R.H. James, P. Bousquet, I. Bussmann, M. Haeckel, R. Kipfer, I. Leifer, H. Niemann, I. Ostrovsky, J. Piskozub, G. Rehder, T. Treude, Limnol. Oceanogr. 61, S283–S299 (2016)
C. Hope, K. Schaefer, Nat. Clim. Change 6, 56–59 (2016)
J.H. van der Waals, J.C. Platteeuw, Adv. Chem. Phys. 2, 1–57 (1959)
A.L. Ballard, E.D. Sloan Jr., Fluid Phase Equilib. 194, 371–383 (2002)
O. Kunz, W. Wagner, J. Chem. Eng. Data 57, 3032–3091 (2012)
J. Gernert, R. Span, J. Chem. Thermodyn. 93, 274–293 (2016)
Calsep, Method documentation PVTsim 20, Calsep product (2011)
Infochem, User guide for multiflash models and physical properties (Infochem Computer Services Ltd., London, 2015)
A.L. Ballard, E.D. Sloan, Fluid Phase Equilib. 218, 15–31 (2004)
http://hydrates.mines.edu/CHR/Software_files/CSMHyd.zip. (1998)
S. Arrhenius, Z. Phys. Chem. 4, 96–116 (1889)
M.C. Clapeyron, Journal de l’École polytechnique 23, 153–190 (1834)
R. Clausius, Ann. Phys. 155, 368–397 (1850)
O.L. Roberts, E.R. Brownscombe, L.S. Howe, Oil Gas J. 39, 37–43 (1940)
W.M. Deaton, E.M. Frost, Gas Hydrates and Their Relation to the Operation of Natural-Gas Pipe Lines, vol. 8, Monograph (United States Bureau of Mines, American Gas Association, Washington, 1946)
A.H. Delsemme, A. Wenger, Planet. Space Sci. 18, 709–715 (1970)
B.J. Falabella, M. Vanpee, Ind. Eng. Chem. Fundam. 13, 228–231 (1974)
T.Y. Makogon, E.D. Sloan Jr., J. Chem. Eng. Data 39, 351–353 (1994)
S.O. Yang, Measurements and predictions of phase equilibria for water + natural gas components in hydrate-forming conditions. Ph.D. Dissertation, Korea University (2000)
A. Hachikubo, A. Miyamoto, K. Hyakutake, K. Abe, H. Shoji, in: Y.H. Mori (ed.), Proceedings of Fourth International Conference on Gas Hydrates, Yokohama (2002)
K. Yasuda, R. Ohmura, J. Chem. Eng. Data 53, 2182–2188 (2008)
A.H. Mohammadi, D. Richon, Ind. Eng. Chem. Res. 49, 3976–3979 (2010)
N. Fray, U. Marboeuf, O. Brissaud, B. Schmitt, J. Chem. Eng. Data 55, 5101–5108 (2010)
H.D. Nagashima, R. Ohmura, J. Chem. Thermodyn. 102, 252–256 (2016)
V.A. Istomin, V.G. Kwon, V.A. Durov, Gas Ind. Rus. 4, 13–15 (2006)
V.P. Melnikov, A.N. Nesterov, A.M. Reshetnikov, A.G. Zavodovsky, Chem. Eng. Sci. 64, 1160–1166 (2009)
S.D. Larson, Phase studies of the two-component carbon dioxide-water system, involving the carbon dioxide hydrate. Ph.D. Dissertation, University of Illinois (1955)
S.L. Miller, W.D. Smythe, Science 170, 531–533 (1970)
A.W. Adamson, B.R. Jones, J. Colloid Interface Sci. 37, 831–835 (1971)
B.J. Falabella, A study of natural gas hydrates. Ph.D. Dissertation, University of Massachusetts, 1975
B. Schmitt, La surface de la glace: structure, dynamique et interactions—implications astrophysique. Ph.D. Dissertation, University of Grenoble (1986)
M. Wendland, H. Hasse, G. Maurer, J. Chem. Eng. Data 44, 901–906 (1999)
A.H. Mohammadi, D. Richon, J. Chem. Eng. Data 54, 279–281 (2009)
H.D. Nagashima, N. Fukushima, R. Ohmura, Fluid Phase Equilib. 413, 53–56 (2016)
V.P. Melnikov, A.N. Nesterov, A.M. Reshetnikov, V.A. Istomin, Chem. Eng. Sci. 66, 73–77 (2010)
Y. Nema, R. Ohmura, I. Senaha, K. Yasuda, Fluid Phase Equilib. 441, 49–53 (2017)
G.J. Chen, T.M. Guo, Chem. Eng. J. 71, 145–151 (1998)
I. Langmuir, J. Am. Chem. Soc. 30, 1361–1403 (1918)
I.V. Yakoumis, G.M. Kontogeorgis, E.C. Voutsas, E.M. Hendriks, D.P. Tassios, Ind. Eng. Chem. Res. 37, 4175–4182 (1998)
A. Martín, J. Phys. Chem. B 114, 9602–9607 (2010)
A. Asiaee, S. Raeissi, A. Shariati, J. Chem. Thermodyn. 43, 822–827 (2011)
I.N. Tsimpanogiannis, N.I. Papadimitriou, A.K. Stubos, Mol. Phys. 110, 1213–1221 (2012)
W.R. Parrish, J.M. Prausnitz, Ind. Eng. Chem. Proc. Des. Dev. 11, 26–35 (1972)
G.D. Holder, G. Corbin, K.D. Papadopoulos, Ind. Eng. Chem. Fundam. 19, 282–286 (1980)
J.B. Klauda, S.I. Sandler, Ind. Eng. Chem. Res. 39, 3377–3386 (2000)
S.Y. Lee, G.D. Holder, AIChE J. 48, 161–167 (2002)
J.B. Klauda, S.I. Sandler, Chem. Eng. Sci. 58, 27–41 (2003)
A.A. Bandyopadhyay, J.B. Klauda, Ind. Eng. Chem. Res. 50, 148–157 (2011)
G. Soave, Chem. Eng. Sci. 27, 1197–1203 (1972)
V. Vinš, A. Jäger, J. Hrubý, R. Span, Fluid Phase Equilib. 435, 104–117 (2017)
G.K. Anderson, J. Chem. Thermodyn. 36, 1119–1127 (2004)
G.K. Anderson, J. Chem. Thermodyn. 35, 1171–1183 (2003)
G. Armstrong, Nat. Chem. 2, 256 (2010)
M.S. Selim, E.D. Sloan, AIChE J. 35, 1049–1052 (1989)
A. Jarrahian, E. Heidaryan, J. Nat. Gas Sci. Eng. 20, 50–57 (2014)
E. Heidaryan, A. Jarrahian, Can. J. Chem. Eng. 91, 1183–1189 (2013)
H.-J. Ng, D.B. Robinson, AIChE J. 23, 477–482 (1977)
E.W. Lemmon, M.L. Huber, M.O. McLinden, NIST reference fluid thermodynamic and transport properties—REFPROP 9.1 (2013)
R. Span, T. Eckermann, S. Herrig, S. Hielscher, A. Jäger, M. Thol, Thermodynamic reference and engineering data—TREND 3.0 (2016)
U. Setzmann, W. Wagner, J. Phys. Chem. Ref. Data 20, 1061–1155 (1991)
R. Span, W. Wagner, J. Phys. Chem. Ref. Data 25, 1509–1596 (1996)
J.H. Yoon, Y. Yamamoto, T. Komai, H. Haneda, T. Kawamura, Ind. Eng. Chem. Res. 42, 1111–1114 (2003)
Y.P. Handa, J. Chem. Thermodyn. 18, 915–921 (1986)
D. Avlonitis, AIChE J. 51, 258–1273 (2005)
M.B. Rydzy, J.M. Schicks, R. Naumann, J. Erzinger, J. Phys. Chem. B 111, 9539–9545 (2007)
R. Feistel, W. Wagner, J. Phys. Chem. Ref. Data 35, 1021–1047 (2006)
A. Gupta, J. Lachance, E.D. Sloan, C.A. Koh, Chem. Eng. Sci. 63, 5848–5853 (2008)
T. Uchida, Waste Manage. 17, 343–352 (1998)
Acknowledgements
The authors appreciate the financial support of FAPESP (processes 2014/02140-7, 2014/25740-0, 2016/09341-3 and 2017/22589-7) and CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heidaryan, E., Robustillo Fuentes, M.D. & Pessôa Filho, P.d.A. Equilibrium of Methane and Carbon Dioxide Hydrates Below the Freezing Point of Water: Literature Review and Modeling. J Low Temp Phys 194, 27–45 (2019). https://doi.org/10.1007/s10909-018-2049-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10909-018-2049-2