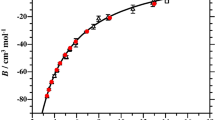
An equation of state for normal liquid 3He has been constructed in the form of Helmholtz free energy as a function of independent parameters—temperature, and density. The equation was fitted simultaneously to the collected experimental p-ρ-T, specific heat, sound velocity, isobaric expansion coefficient and isothermal compressibility coefficient from the world’s literature to accuracies comparable with reasonable experimental errors in the measured quantities. Extensive comparisons between the equation of state and experimental data have been made by a set of deviation plots. The state equation is valid in the region for temperatures from 0.1 K to T c = 3.3157 K, and for pressures from vapor pressures to melting pressures.
Similar content being viewed by others
References
Kollar M., Vollhardt D, (2000). Phys. Rev. B 61, 15347
Sherman R.H., Edeskuty F.J, (1960). Ann. Phys 9, 522
Kerr E.C., Taylor R.D, (1962). Ann. Phys. 20, 450
Greywall D.S, (1983). Phys. Rev B 27: 2747
Greywall D.S, (1986). Phys. Rev 33: 7520
Huang Y.H, Chen G.B, Li X.Y, Arp V.D, (2005). Int. J. Thermophys. 26, 1
Bogoyavlenskii I.V., Yurchenko S.I, (1976). Sov. J. Low Temp. Phys. 2, 672
Kerr E.C., Sherman R.H, (1970). J. Low. Temp. Phys. 3, 451
Grilly E.R, Mills R.L, (1959). Ann. Phys 8: 1
Grilly E.R, (1971). J. Low Temp. Phys. 4, 615
Roberts T.R., Sydoriak S.G, (1954). Phys. Rev. 93: 1418
Roberts T.R, Sydoriak S.G, (1955). Phys. Rev. 98: 1672
Huang Y.H, Chen G.B, Cryogenics (in Chinese) 146, 1 (2005).
Reesink A.L., Durieux M, (1996). Metrologia 33, 401
Rusby R.L, Durieux M, Reesink A.L, Hudson R.P, Schuster G, Kühne M., Fogle W.E, Soulen R.J, Adams E.D, (2003). AIP Conf Proc. 684, 77
Abraham B.M, Osborne D.W, (1971). J. Low Temp. Phys. 5, 335
Wallace B.Jr., Meyer H, (1970). Phys. Rev A 2: 1563
Laquer H.L, Sydoriak S.G, Roberts T.R, (1959). Phys. Rev 113, 417
Atkins K.R., Flicker H, (1959). Phys. Rev 113, 959
Atkins K.R., Flicker H, (1959). Phys. Rev 116: 1063
Vignos J.H, Fairbank H.A, (1966). Phys. Rev 147, 185
Schmidt R., Wagner W, (1985). Fluid Phase Equilibria 19, 175
Jacobsen R.T, Penoncello S.G, Lemmon E.W, (1997). Thermodynamic Properties of Cryogenic Fluids. Plenum Press, New York
Boghosian C, Meyer H, Rives J.E, (1966). Phys. Rev. 146, 110
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Y., Chen, G., Wang, S. et al. Equation of State for Normal Liquid Helium-3 from 0.1 to 3.3157 K. J Low Temp Phys 143, 1–29 (2006). https://doi.org/10.1007/s10909-006-9208-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10909-006-9208-6