Abstract
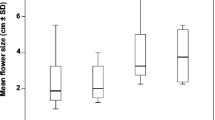
In considerations of sexual floral size dimorphism, there is a conflict between sexual selection theory, which predicts that larger floral displays attract more pollinators, and optimality theory—particularly the ideal free distribution—which predict that pollinators' visits should match nutritional rewards. As an alternate explanation of this dimorphism, Müller reported that pollinators tend to visit larger male flowers before visiting smaller female flowers, thereby promoting effective pollination. To investigate optimality predictions, I offered pollinators a choice between smaller, less numerous, but more rewarding flowers; and larger, more numerous, but less rewarding flowers. Foragers initially favored the larger and more numerous flowers, but rapidly shifted preferences to conform with the predictions of the ideal free distribution. To test Müller's hypothesis, I offered pollinators choices between larger and smaller corollas of equal caloric reward. Results showed that although pollinators tended to visit larger corollas first, they did not visit them more often. These experiments highlight the need for further investigation into the tradeoff between natural and sexual selection, and their respective influences in pollination ecology.
Similar content being viewed by others
References
Abraham, J. N. (1996). La saboteuse: An ecological theory of sexual dimorphism in animals. Acta Biotheor. 46: 23–35.
Agren, J., Elmqvist, T., and Tunlid, A. (1986). Pollination by deceit, floral sex ratios and seed set in dioecious Rubus chamaemorus L. Oecologia 70: 332–338.
Andersson, S. (1991). Floral display and pollination success in Achillea ptarmica (Asteraceae). Hol. Ecol. 14: 186–191.
Anderson, G. J., and Symon, D. E. (1989). Functional dioecy and andromonoecy in Solanum. Evolution 43: 204–219.
Arnold, S. J. (1994). Supplement: Sexual selection in plants and animals, a symposium organized by Stevan J. Arnold. Am. Nat. 144: S1–S149.
Ashman, T. L., and Stanton, M. L. (1991). Seasonal variation in pollination dynamics of sexually dimorphic Sidalcea oregana ssp. spicata (Malvaceae). Ecology 72: 993–1003.
Baker, H. G. (1948). Corolla size in gynodioecious and gynomonoecious species of flowering plants. Proc. Leeds Phil. Lit. Soc. 5: 136–139.
Bawa, K. S. (1980a). Mimicry of male by female flowers and intrasexual competition for pollinators in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae). Evolution 34: 467–474.
Bawa, K. S. (1980b). Evolution of dioecy in flowering plants. Ann. Rev. Ecol. Syst. 11: 15–39.
Beach, J. H. (1981). Pollinator foraging and the evolution of dioecy. Am. Nat. 118: 572–577.
Bell, G. (1985). On the function of flowers. Proc. R. Soc. Lond. B 224: 223–265.
Bell, G., Lefebvre, L., Giraldeau, L.-A., and Weary, D. (1984). Partial preference of insects for the male flowers of an annual herb. Oecologia 65: 287–294.
Broyles, S. B., and Wyatt, R. (1995). A reexamination of the pollen-donation hypothesis in an experimental population of Asclepias exaltata. Evolution 49: 89–99.
Broyles, S. B., and Wyatt, R. (1997). The pollen donation hypothesis revisited: A response to Queller. Am. Nat. 149: 595–599.
Butler, C. G. (1945). The influence of various physical and biological factors of the environment on honey bee activity. An examination of the relationship between activity and nectar concentration and abundance. J. Exp. Biol. 21: 5–12.
Campbell, D. R. (1989). Inflorescence size: A test of the male function hypothesis. Am. J. Bot. 76: 730–738.
Campbell, D. R., Waser, N. M., Price, M. V., Lynch, E. A., and Mitchell, R. J. (1991). Components of phenotypic selection: Pollen export and flower corolla width in Ipomopsis aggregata. Evolution 45: 1458–1467.
Chaplin, S. J., and Walker, J. L. (1982). Energetic constraints and adaptive significance of the floral display of a forest milkweed. Ecology 63: 1857–1870.
Comba, L., Corbet, S. A., Barron, A., Bird, A., Collinge, S., Miyazaki, N., and Powell, M. (1999). Garden flowers: insect visits and the floral reward of horticulturally-modified variants. Ann. Bot. 83: 73–86.
Conner, J. K., and Rush, S. (1996). Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum. Oecologia 104: 409–516.
Corbet, S. A., Cuthill, I., Fallows, M., Harrison, T., and Hartley, G. (1981). Why do nectar-foraging bees and wasps work upwards on inflorescences? Oecologia 51: 79–83.
Dafni, A. (1984). Mimicry and deception in pollination. Ann. Rev. Ecol. Syst. 15: 359–378.
Darwin, C. (1859). On the Origin of Species by Means of Natural Selection. J. Murray, London.
Darwin, C. (1871). The Descent of Man and Selection in Relation to Sex. J. Murray, London.
Darwin, C. (1888). The Different Forms of Flowers on Plants of the Same Species, University of Chicago Press, Chicago.
Delph, L. F., and Lively, C. M. (1992). Pollinator visitation, floral display, and nectar production of the sexual morphs of a gynodioecious shrub. Oikos 63: 161–170.
Devlin, B., and Stephenson, A. G. (1985). Sex differential floral longevity, nectar secretion, and pollinator foraging in a protandrous species. Am. J. Bot. 72: 303–310.
Dreisig, H. (1995). Ideal free distributions of nectar foraging bumblebees. Oikos 72: 161–172.
Eckhart, V. M. (1991). The effects of floral display on pollinator visitation vary among populations of Phacelia linearis (Hydrophyllaceae). Evol. Ecol. 5: 370–384.
Emms, S. K., Stratton, D. A., and Snow, A. A. (1997). The effect of inflorescence size on male fitness: experimental tests in the andromonoecious lily, Zigadenus paniculatus. Evolution 51: 1481–1489.
Faegri, K., and van der Pijl, L. (1979). The Principles of Pollination Ecology, 3rd revised ed. Pergamon, Oxford.
Fisher, R. A. (1915). The evolution of sexual preference. Eugen. Rev. 7: 184–192.
Fisher, R. A. (1958). The Genetical Theory of Natural Selection, Dover, New York.
Firmage, D. H., and Cole, F. R. (1988). Reproductive success and inflorescence size of Calopogon tuberosus (Orchidaceae). Am. J. Bot. 75: 1371–1377.
Frankie, G. W., and Haber, W. A. (1983). Why bees move among mass flowering neotropical trees. In Jones, C. E., and Little, R. J. (eds.), Handbook of Experimental Pollination Biology. Scientific and Academic Editions, New York, pp. 360–372.
Free, J. B. (1968). Dandelion as a competitor to fruit trees for bee visits. J. Appl. Ecol. 5: 169–178.
Grant, K. (1966). A hypothesis concerning the prevalence of red coloration in California hummingbird flowers. Am. Nat. 100: 85–97.
Grant, K. (1995). Sexual selection in plants: Pros and cons. Proc. Nat. Acad. Sci. USA 92: 1247–1250.
Harder, L. D., and Cruzan, M. B. (1990). An evaluation of the physiological and evolutionary influences of inflorescence size and flower depth on nectar production. Funct. Ecol. 4: 559–572.
Harder, L. D., Thomson, J. D., Cruzan, M. B., and Unnasch, R. S. (1985). Sexual reproduction and variation in floral morphology in an ephemeral vernal lily, Erythronium americanum. Oecologia 67: 286–291.
Haynes, J. G., and Mesler, M. (1984). Pollen foraging by bumblebees: Foraging patterns and efficiency of Lupinus polyphyllus. Oecologia 61: 249–253.
Heinrich, B. (1975). Energetics of pollination. Ann. Rev. Ecol. Syst. 6: 139–170.
Heinrich, B. (1976). The foraging specializations of individual bumblebees. Ecol. Mon. 46: 105–128.
Heinrich, B. (1983). Insect foraging energetics. In Jones, C. E., and Little, R. J. (eds.), Handbook of Experimental Pollination Biology. Scientific and Academic Editions, New York, pp. 187–214.
Huxley, J. S. (1938). Darwin's theory of sexual selection and the data subsumed by it, in the light of recent research. Am. Nat. 72: 416–433.
Janzen, D. H. (1971). Euglossine bees as long distance pollinators of tropical plants. Science 171: 203–205.
Janzen, D. H. (1977). A note on optimal mate selection by plants. Am. Nat. 111: 365–371.
Kaplan, S. M., and Mulcahy, D. L. (1971). Mode of pollination and floral sexuality in Thalictrum. Evolution 25: 659–668.
Kay, Q. O. N., Lack, A. J., Bamber, F. C., and Davies, C. R. (1984). Differences between sexes in floral morphology, nectar production and insect visits in a dioecious species. Silene dioica. New Phytol. 98: 515–529.
Krebs, J. R., and Davies, N. B. (1993) An Introduction to Behavioural Ecology, Blackwell Scientific, Oxford.
Lloyd, D. G., and Webb, C. J. (1977). Secondary sex characters in plants. Bot. Rev. 43: 177–216.
Lyons, E. E., Waser, N. M., Price, M. V., Antonovics, J., and Motten, A. F. (1989). Sources of variation in plant reproductive success and implications for concepts of sexual selection. Am. Nat. 134: 409–433.
Maynard Smith, J. (1991). Theories of sexual selection. Trends Ecol. Evol. 6: 146–151.
McGregor, S. E., Alcorn, S. M., Kurtz, E. G. Jr., and Butler, G. D. Jr. (1959). Bee visitors to saguaro flowers. J. Econ. Entom. 52: 1002–1004.
Mitchell, R. J. (1993). Adaptive significance of Ipomopsis aggregata nectar production: Observation and experiment in the field. Evolution 47: 25–35.
Miyake, T., and Yafuso, M. (2003). Floral scents affect reproductive success in fly-pollinated Alocasia odora (Araceae). Am. J. Botany 90: 370–376.
Müller, H. (1873). Ground ivy. Nature 8: 161–162.
Nakamura, R. R., Stanton, M. L., and Mazer, S. J. (1989). Effects of mate size and mate number on male reproductive success in plants. Ecology 70: 71–76.
Pleasants, J. M. (1981). Bumblebee response to variation in nectar availability. Ecology 62: 1648–1661.
Poldolsky, R. D. (1993). Evolution of a flower dimorphism: How effective is pollen dispersal by “male” flowers? Ecology 74: 2255–2260.
Primack, R. B. (1987). Relationships among flowers, fruits, and seeds. Ann. Rev. Ecol. Syst. 18: 409–430.
Pyke, G. H. (1978). Optimal foraging: Movement patterns of bumblebees between inflorescences. Theor. Pop. Biol. 13: 72–98.
Pyke, G. H. (1982). Foraging in bumblebees: Rule of departure from an inflorescence. Canadian J. of Zool. 60: 417–428.
Pyke, G. H., Pulliam, H. R., and Charnov, E. L. (1977). Optimal foraging: A selective review of theory and tests. Q. Rev. Biol. 52: 137–154.
Queller, D. C. (1983). Sexual selection in a hermaphroditic plant. Nature 305: 706–707.
Ryan, M. J., and Rand, A. S. (1990). The sensory basis of sexual selection for complex calls in the túngara frog, Physalae mus pustulosus (sexual selection for sensory exploitation). Evolution 44: 305–314.
Spaethe, J., Tautz, J., and Chittka, L. (2001). Visual constraints in foraging bumblebees: Flower size and color affect search time and flight behavior. Proc. Nat. Acad. Sci. 98: 3898–3903.
Stanton, M. L., Young, H. J., Ellstrand, N. C., and Clegg, J. M. (1991). Consequences of floral variation for male and female reproduction in experimental populations of wild radish, Raphanus sativus L. Evolution 45: 268–280.
Stanton, M. L., Ashman, T.-L., Galloway, L. F., and Young, H. J. (1992). Estimating male fitness of plants in natural populations. In Wyatt, R. (ed.), Ecology and Evolution of Plant Reproduction, Chapman& Hall, New York, pp. 62–90.
Stephenson, A. G., and Bertin, R. I. (1983). Male competition, female choice, and sexual selection in plants. In Real, L. (ed.), Pollination Biology, Academic Press, New York, pp. 110–149.
Sutherland, S., and Delph, L. F. (1984). On the importance of male fitness in plants: Patterns of fruit set. Ecology 65: 1093–1104.
Thomson, J. D., and Plowright, R. C. (1980). Pollen carryover, nectar rewards, and pollinator behavior with special reference to Diervilla lonicera. Oecologia 46: 68–74.
Thomson, J. D., Maddison, W. P., and Plowright, R. C. (1982). Behavior of bumble bee pollinators of Aralia hispida Vent. (Araliaceae). Oecologia 54: 326–336.
Thomson, J. D., McKenna, M. A., and Cruzan, M. B. (1989). Temporal patterns of nectar and pollen production in Aralia hispida: Implications for reproductive success. Ecology 70: 1061–1068.
Waddington, D., and Heinrich, B. (1979). The foraging movements of bumblebees on vertical inflorescences: An experimental analysis. J. Comp. Physiol. 134: 113–117.
Wainselboim, A. J., Roces, F., and Farina, W. M. (2002). Honeybees assess changes in nectar flow within a single foraging bout. Animal Behav. 63: 1–6.
Willson, M. F. (1979). Sexual selection in plants. Am. Nat. 113: 777–790.
Willson, M. F. (1990). Sexual selection in plants and animals. Trends Ecol. Evol. 5: 210–214.
Willson, M. F. (1991). Sexual selection, sexual dimorphism and plant phylogeny. Evol. Ecol. 5: 69–87.
Willson, M. F. (1994). Sexual selection in plants: Perspective and overview. Am. Nat. 144: S13–S39.
Willson, M. F., and Rathcke, B. J. (1974). Adaptive design of the floral display in Asclepias syriaca L. Am. Mid. Nat. 92: 47–57.
Wilson, P., Thomson, J. D., Stanton, M. L., and Rigney, L. P. (1994). Beyond floral Batemania: Gender biases in selection for pollination success. Am. Nat. 143: 283–296.
Young, H. J., and Stanton, M. L. (1990). Influences of floral variation on pollen removal and seed production in wild radish. Ecology 71: 536–547.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abraham, J.N. Insect Choice and Floral Size Dimorphism: Sexual Selection or Natural Selection?. J Insect Behav 18, 743–756 (2005). https://doi.org/10.1007/s10905-005-8737-1
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10905-005-8737-1