Abstract
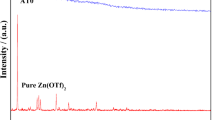
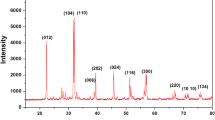
The present work reports the development of nanosized multiferroic filler BiFeO3 impregnated PVA based nanocomposite gel polymer electrolyte (NCGPEs) through conventional solution cast technique. The gel films have been characterized by Fourier Transform Infrared Spectroscopy (FTIR), X-Ray diffraction (XRD), Thermogravimetric Analysis (TGA), Differential Scanning Calorimetry (DSC) and Electrochemical Impedance Spectroscopy (EIS) to ascertain their performance in electrochemical devices. FTIR studies confirmed complexation of salt with polymer matrix and change in morphology upon addition of BiFeO3 fillers. Enhancement in glass transition temperature with increasing filler content was observed in DSC studies. Filler inclusion increased thermal stability of the system according to TGA results. High value of ionic transference number (0.99) reveals ionic conduction. The NCGPEs sample [PVA (35): NH4CH3 COO(65)]:0.5wt%BiFeO3 exhibited highest ionic conductivity (1.05X10−3Scm−1). Charge carrier concentration seems to be responsible for high ionic conduction. CV&LSV measurement indicate the applicability of electrolyte in energy storage applications.

















Similar content being viewed by others
References
Y. Liao, M. Rao, W. Li, C. Tan, J. Yi, L. Chen, Improvement in ionic conductivity of self-supported P(MMA-AN-VAc) gel electrolyte by fumed silica for lithium ion batteries. Electrochim. Acta 54(26), 6396–6402 (2009)
F. Chen, X. Ma, X. Qu, H. Yan, Structure and properties of an organic rectorite/poly(methyl methacrylate) nanocomposite gel polymer electrolyte by in situ synthesis. J. Appl. Polym. Sci. 114(5), 2632–2638 (2009)
O. Krejza, J. Velická, M. Sedlaříková, J. Vondrák, The presence of nanostructured Al2O3 in PMMA-based gel electrolytes. J. Power Sources 178(2), 774–778 (2008)
Y.J.H. Chen, M. Chen, N. Liu, L. Qingwen, Graphene-Patched CNT/MnO2 nanocomposite papers for the electrode of high-performance flexible asymmetric supercapacitors. ACS Appl Mater. Interfaces 5(8), 3408–3416 (2013). https://doi.org/10.1021/am400457x
S. Alipoori, S. Mazinani, S.H. Aboutalebi, F. Sharif, Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 27, 101072 (2020)
J. Wang, Z. Zhao, S. Song, Q. Ma, R. Liu, High performance poly(vinyl alcohol)-based li-ion conducting gel polymer electrolyte films for electric double-layer capacitors. Polymers (Basel). 10(11), 1179 (2018)
S.L. Agrawal, N. Rai, DMA and conductivity studies in PVA:NH4SCN:DMSO:MWNT nanocomposite polymer dried gel electrolytes. Hindawi Publishing Corporation J. Nanomater. 2015, 1–7 (2015). https://doi.org/10.1155/2015/435625
J. Wang, S. Song, R. Muchakayala, X. Hu, R. Liu, Structural, electrical, and electrochemical properties of PVA-based biodegradable gel polymer electrolyte membranes for Mg-ion battery applications. Ionics 23, 1759–1769 (2017). https://doi.org/10.1007/s11581-017-1988-y
T.C. Zhou, J. Zhang, J.L. Qiao, L.L. Liu, G.P. Jiang, J. Zhang, Y.Y. Liu, High durable poly(vinyl alcohol)/Quaterized hydroxyethylcellulose ethoxylate anion exchange membranes for direct methanol alkaline fuel cells. J. Power. Sources 227, 291–299 (2013)
Y. Xiong, Q.L. Liu, Q.G. Zhang, A.M. Zhu, Synthesis and characterization of cross-linked quaternized poly (vinyl alcohol)/chitosan composite anion exchange membranes for fuel cells. J. Power. Sources 183, 447–453 (2008)
C.P. Singh, P.K. Shukla, S.L. Agrawal, Ion transport studies in PVA:NH4CH3COO gel polymer electrolytes. High Performance Polymer 32, 208–219 (2020)
C.P. Lovely Ranjta, N.R. Singh, Experimental investigations on nano-ferrite embedded nanocomposite polymer electrolytes for proton-conducting rechargeable batteries application. Mater.Today: Proc. 54, 702–709 (2022)
B.D. Cullity, Elements of X-Ray Diffraction, 2nd edn. (Addison-Wesley Publishing, Boston, 1956)
P. Scherrer, Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen, Mathematisch-Physikalische Klasse. 2, 98–100 (1918)
K. Chaurasia Sujeet, K. Singh Rajendra, S. Chandra, Dielectric relaxation and conductivity studies on (PEO:LiClO4) polymer electrolyte with added ionic liquid [BMIM][PF6]: evidence of ion-ion interaction. J. Polym. Sci. 49, 291–300 (2011)
C.P. Neelesh Rai, L.R. Singh, Structural, thermal and electrical studies of Al 2 O 3 nanoparticle soaked electrolyte gel films for novel proton conducting (H + ion) eco-friendly device applicationsamerican. J. Nano Res. App. 10(1), 1–8 (2022)
G.X. Zou, P.Q. Jin, L.Z. Xin, Extruded Starch/PVA Composite: Water resitance, thermal properties and morphology. J Elastomers Plastic 40, 303–316 (2008)
A. Awadhia, S.L. Agrawal, Structural, thermal and electrical characterizations of PVA: DMSO:NH4SCN Gel Electrolyte. Solis State Ionic 178, 951–958 (2007)
L. Ranjta, C.P. Singh, N. Rai, Experimental investigation on nano- ferrite embedded nanocomposite polymer electrolyte for proton conducting rechargeable batteries application. Mater. Today Proc. 54(3), 702–709 (2022)
S.L. Agrawal, P.K. Shukla, D. Tripathi, C.P. Singh, Studies on multiferroic oxide-doped PVA-based nanocomposite gel polymer electrolyte system for electrochemical device application. Ionics 25(2), 617 (2019). https://doi.org/10.1007/s11581-018-2635-y
G. Tsagaropoulos, A. Eisenberg, Dynamic mechanical study of the factors affecting the two glass transition behavior of filled polymers. Similarities and differences with random ionomers. Macromolecules 28, 6067–6077 (1995)
J. Adebahr, A.S. Best, N. Byrne, P. Jacobsson, D.R. MacFarlane, M. Forsyth, Ion transport in polymer electrolytes containing nanoparticulate TiO2: the influence of polymer morphology. Phys. Chem. Chem. Phys. 5, 720–725 (2003)
B. Kumar, Heterogeneous electrolytes: variables for and uncertainty in conductivity measurements. J. Power. Sources 179, 401–406 (2008)
A.J. Bhattacharyya, J. Maier, Second phase effects on the conductivity of non-aqueous salt solutions: B Soggy sand electrolytes. Adv. Mater. 16, 811–814 (2004)
Y. Tominaga, S. Asai, M. Sumita, S. Panero, B. Scrosati, A novel composite polymer electrolyte: effect of mesoporous SiO2 on ionic conduction in poly(ethylene oxide)–LiCF3SO3 complex. J Power Sources 146, 402–40 (2005)
S.A. Suthanthiraraj, R.J. Kumar, B. Paul, Ionics 16, 145–151 (2009)
F.B. Dias, S.V. Batty, J.P. Voss, G. Ungar, P.V. Wright, Ionic conductivity of a novel smectic polymer electrolyte. Solid State Ionics 85, 43–49 (1996)
C.-W. Liew, K.H. Arifin, J. Kawamura et al., Electrical and structural studies of ionic liquid- based poly (vinyl alcohol) proton conductors. J. Non. –Cryst. Solid 425, 163–172 (2015)
A.L. Saroj, R.K. Singh, J. Phys. Chem. Solids 73, 162 (2012)
M.M. Abutalib, Phys. B 557, 108 (2019)
R. Kumar, N. Arora, S. Sharma, N. Dhiman, D. Pathak, Ionics 23, 2761 (2017)
Acknowledgements
The authors are thankful to the Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Large Research Group Project under Grant no. R.G.P.2/557/44. Also, this work is partially supported by the co-mentor Prof. Kaushik Pal who is grateful to Department of Science and Technology, New Delhi, Govt. of India funded scheme (DST/WoS-A/CS-49/2021) WOS-A to his post-doctoral scholar Dr. Nidhi Asthana providing external research support.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
C. P. Singh1- Write original draft and spectroscopic experimental investigations P. K. Shukla- Investigation of samples preparation and review data analysis Kaushik Pal- Direction of research plan, data investigations, review the draft writing Nidhi Asthana- Writing draft and experimental analysis Anshuman Srivastava- Resources of samples preparation and original draft preparation S. L. Agrawal- Data analysis and experiments Safia Obaidur Rab- Funding recourses and review the draft Saad Alamri- Funding recourses and review the draft
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Development of nanosized multiferroic filler BifeO3 impregnated PVA based nanocomposite gel polymer electrolyte (NCGPEs) through conventional solution cast technique.
• The NCGPEs sample [PVA (35): NH4CH3 COO (65)]:0.5wt% BFO exhibited highest ionic conductivity (1.05X10−3Scm−1). Charge carrier concentration seems to be responsible for high ionic conduction.
• CV&LSV measurement indicate the applicability of electrolyte in energy storage applications.
• BFO is one of the promising materials due to its high dielectric constant below the Curie temperature.
• It has been asserted that the addition of BiFeO3 (BFO) creates additional hopping site for the charge carriers and hence increases its concentration to result in enhancement of conductivity.
• Linear Sweep and Cyclic Voltammetric investigations have shown that BiFeO3 impregnated NCPEs have good window stability (5.43 V).
• The compositional dependence has been correlated to charge carrier concentration at lower filler concentrations, improvement in system morphology (amorphous behavior) and association/dissociation effect in accordance with breathing chain model.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, C.P., Shukla, P.K., Pal, K. et al. Structural, Thermal, Electrical and Electrochemical Studies of Polyvinyl Alcohol (PVA) Assisted BiFeO3 Embedded Novel Gel-Based Nanocomposite Utilizations in Polymer Electrolytes. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03085-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03085-5