Abstract
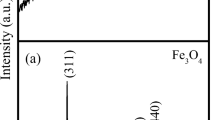
A facile hydrothermal-calcination process was employed to fabricate Fe3O4/α-Fe2O3 heterogeneous nanosheets utilizing NH4H2PO4 as the restricted growth agent and FeCl3 as the iron source. The morphologies and properties of the obtained nanomaterials were investigated by SEM, XRD, VSM, and TEM techniques. In the hydrothermal process, an H2PO4− concentration of 1.44 mM, Fe3+ concentration of 20 mM, a hydrothermal temperature of 220 °C, and a hydrothermal time of 24 h were selected as the optimal conditions for α-Fe2O3 nanosheets, their average diameter and thickness of the resulting nanosheets were approximately 150 and 53 nm. Subsequently, glucose was utilized as a reducing agent to partially reduce the precursor of α-Fe2O3 to Fe3O4, thereby forming Fe3O4/α-Fe2O3 heterogeneous nanosheets. During the calcination process, the effects of calcination time, calcination temperature, and glucose content on the morphology and properties of the nanosheets were investigated. The morphology and size of the prepared nanosheets did not change significantly during the calcination process, while the magnetic properties of the products underwent significant changes. When the product was calcined at 600 °C for 4 h with a mass ratio of precursor to glucose was 1:12, the saturation magnetization of as-prepared Fe3O4/α-Fe2O3 heterogeneous nanosheets reached the largest of 80 emu/g. The results indicated that the Fe3O4/α-Fe2O3 heterogeneous nanosheets with satisfactory saturation magnetization were successfully fabricated with glucose as the reductant.
Graphical Abstract













Similar content being viewed by others
References
R. Brüngel, J. Rückert, P. Müller et al., Nanodefiner framework and e-tool revisited according to the European commission’s nanomaterial definition 2022/C 229/01. Nanomaterials 13(6), 990 (2023). https://doi.org/10.3390/nano13060990
M. Shin, J. Lim, J. An et al., Nanomaterial-based biohybrid hydrogel in bioelectronics. Nanoconverg 10, 8 (2023). https://doi.org/10.1186/s40580-023-00357-7
Y.O. Donar, A.T. Ünalan, S. Ergenekon et al., Catalytic evaluation of cellulose pyrolysis by using nanosized metal oxide catalysts. Turk. J. Chem. 47, 116–125 (2023). https://doi.org/10.55730/1300-0527.3522
B. Budak, S. Demi̇Rel, Synthesis and characterization of PANI and PANI/nanometal oxides, photocatalytic and adsorbent applications. Turk. J. Chem. 47, 346–363 (2023). https://doi.org/10.55730/1300-0527.3542
L. Zhang, G. Tian, F. Wu et al., The influence of grain size and catalytic doping on magnesium hydride nanocrystals for hydrogen storage. J. Phys. Chem. Solids 178, 111335 (2023). https://doi.org/10.1016/j.jpcs.2023.111335
H. Xiang, S. Xu, W. Zhang et al., Skin permeation of curcumin nanocrystals: effect of particle size, delivery vehicles, and permeation enhancer. Colloid. Surf. B 224, 113203 (2023). https://doi.org/10.1016/j.colsurfb.2023.113203
M. Elhamel, Z. Hebboul, M.E. Naidjate et al., Experimental synthesis of double perovskite functional nano-ceramic Eu2NiMnO6: combining optical characterization and DFT calculations. J. Solid. State Chem. 323, 124022 (2023). https://doi.org/10.1016/j.jssc.2023.124022
H. Dai, Q. Shen, J. Chen et al., Improving the magnetic and dielectric properties of Cu1 – Ho FeO2 nanoceramics by tuning the vacancy defects and fe valence state. Ceram. INT 49(10), 16451–16457 (2023). https://doi.org/10.1016/j.ceramint.2023.02.006
Z. Sui, Y. Sun, Y. Jing et al., Robust CsPbBr3 and Zn-Cd-S quantum dots co-doped nano-glass composites with broadly tunable emissions. J. Eur. Ceram. Soc. 43(4), 1683–1688 (2023). https://doi.org/10.1016/j.jeurceramsoc.2022.11.062
K. Vinzant, M. Rashid, M.V. Khodakovskaya, Advanced applications of sustainable and biological nano-polymers in agricultural production. Front. Plant Sci. 13, 1081165 (2023). https://doi.org/10.3389/fpls.2022.1081165
L. Doan, L.T. Nguyen, N.T.N. Nguyen, Modifying superparamagnetic iron oxides nanoparticles for doxorubicin delivery carriers: a review. J. Nanopart Res. 25, 73 (2023). https://doi.org/10.1007/s11051-023-05716-3
M. Baghiat Esfahani, A. Khodavandi, F. Alizadeh et al., Biofilm-associated genes as potential molecular targets of nano-Fe3O4 in Candida albicans. Pharmacol. Rep. 75, 682–694 (2023). https://doi.org/10.1007/s43440-023-00467-3
N. Zhang, Y. Guo, H. Huang et al., Onset potential shift of water oxidation in the metastable phase transformation process of β-Fe2O3. Energy Fuels 36(19), 11567–11575 (2022). https://doi.org/10.1021/acs.energyfuels.2c01812
S. Wang, X. Chen, L. Bao et al., A magnetic Fe3O4/modified bentonite composite as recyclable heterogeneous catalyst for synthesizing 2-substituted benzimidazoles. Chem. Select. 8(13), e202204930 (2023). https://doi.org/10.1002/slct.202204930
K. Cui, L. Yuan, Z. Zhao, Magnetic properties of Ni3Si/Fe3O4@PVDF composites with different Fe3O4 nanoparticles content based on lamellar Ni3Si template. Mat. Sci. Eng. B-Adv. 290, 116330 (2023). https://doi.org/10.1016/j.mseb.2023.116330
B.G. Kim, J. Park, W. Choi et al., Electrocatalytic arsenite oxidation using iron oxyhydroxide polymorphs (α-, β-, and γ-FeOOH) in aqueous bicarbonate solution. Appl. Catal. B-Environ. 283, 119608 (2021). https://doi.org/10.1016/j.apcatb.2020.119608
B. Hu, X. Yan, W. Wang et al., Iron oxyhydroxide polytype (γ-, δ- and β-FeOOH) structures govern zn mobility. Chem. Geol. 614, 121167 (2022). https://doi.org/10.1016/j.chemgeo.2022.121167
J. Wang, M. Liu, Y. Ni et al., Fabrication and characterization of magnetic α-Fe2O3/Fe3O4 heterogeneous nanosheets. J. Inorg. Organomet P 33, 783–795 (2023). https://doi.org/10.1007/s10904-023-02536-9
Z.A. Hamid, M.H. Gomaa, S.I. El-Hout et al., α-Fe2O3/Fe3O4@GO nanosheets boost the functionality of Ni P thin film deposited by electroless method. Surf. Coat. Tech. 456, 129288 (2023). https://doi.org/10.1016/j.surfcoat.2023.129288
N.L. Tuyen, T.Q. Toan, N.B. Hung et al., Simultaneous precipitation and discharge plasma processing for one-step synthesis of α-Fe2O3–Fe3O4/graphene visible light magnetically separable photocatalysts. RSC Adv. 13, 7372–7379 (2023). https://doi.org/10.1039/D2RA06844C
R. Liu, G. Rong, Y. Liu et al., Delivery of apigenin-loaded magnetic Fe2O3/Fe3O4@mSiO2 nanocomposites to A549 cells and their antitumor mechanism. Mat. Sci. Eng. C 120, 111719 (2021). https://doi.org/10.1016/j.msec.2020.111719
R. Liu, Y. Zhang, P. Deng et al., Construction of targeted delivery system for curcumin loaded on magnetic α-Fe2O3/Fe3O4 heterogeneous nanotubes and its apoptosis mechanism on MCF-7 cell. Biomater. Adv. 136, 212783 (2022). https://doi.org/10.1016/j.bioadv.2022.212783
Y. Ni, H. Ouyang, L. Yu et al., Label-free electrochemical aptasensor based on magnetic α-Fe2O3/Fe3O4 heterogeneous hollow nanorods for the detection of cancer antigen 125. Bioelectrochemistry 148, 108255 (2022). https://doi.org/10.1016/j.bioelechem.2022.108255
Y. Ni, X. Chen, C. Ling et al., Electrochemical peptide nucleic acid functionalized α-Fe2O3/Fe3O4 nanosheets for detection of CYP2C19*2 gene. Microchim. Acta 190, 189 (2023). https://doi.org/10.1007/s00604-023-05781-4
N. Sakono, Y. Ishida, K. Ogo et al., Molar-fraction-tunable synthesis of Ag–Au Alloy Nanoparticles via a dual evaporation–condensation method as supported catalysts for CO oxidation. ACS Appl. Nano Mater. 6(4), 3065–3074 (2023). https://doi.org/10.1021/acsanm.3c00089
X. Liu, L. Zhang, Z. Li, Promotion effect of TiO2 on Ni/SiO2 catalysts prepared by hydrothermal method for CO2 methanation. Energy Technol-Ger. 11(7), 2201526 (2023). https://doi.org/10.1002/ente.202201526
P. Saraswathi, S. Madeswaran, Formation of nanostructured Fe88Co12 alloy using high energy ball milling. J. Magn. Magn. Mater. 560, 169652 (2022). https://doi.org/10.1016/j.jmmm.2022.169652
Y. Huo, Y. Zhu, J. Xie et al., Controllable synthesis of hollow α-Fe2O3 nanostructures, their growth mechanism, and the morphology-reserved conversion to magnetic Fe3O4/C nanocomposites. RSC Adv. 3, 19097–19013 (2013). https://doi.org/10.1039/c3ra42764a
Y. Yang, X. Liu, J. Ding, Synthesis of α-Fe2O3 templates via hydrothermal route and Fe3O4 particles through subsequent chemical reduction. Sci. Adv. Mater. 5(9), 1199–1207 (2013). https://doi.org/10.1166/sam.2013.1573
Acknowledgements
The authors wish to thank the Jiangsu Provincial Postgraduate Scientific Practice and Innovation Project (Grant No. SJCX21_1722) and the Science and Technology Innovation Project of CHN Energy (Grant No. GJNY-20-109).
Funding
This work was supported by the Jiangsu Provincial Postgraduate Scientific Practice and Innovation Project (Grant No. SJCX21_1722) and the Science and Technology Innovation Project of CHN Energy (Grant No. GJNY-20-109).
Author information
Authors and Affiliations
Contributions
JW conducted material preparation, data acquisition and analysis, and wrote the first draft of the paper. HO made substantial contributions to the conception or designed of the study and interpretation of data in the study. YN participated in the writing of the first draft of the paper, and the conception and operation of the experimental designed. NH and YY reviewed, revised and edited the article. DZ designed and guided the experimental operation, and put forward rectification suggestions for the article. YL supervised the entire experimental study.
Corresponding authors
Ethics declarations
Conflict of interest
The author declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Ouyang, H., Ni, Y. et al. A Hydrothermal-Calcination Process with Ammonium Dihydrogen Phosphate as Restricted Growth Agent for the Fabrication of Magnetic Fe3O4/α-Fe2O3 Heterogeneous Nanosheets. J Inorg Organomet Polym 34, 1015–1027 (2024). https://doi.org/10.1007/s10904-023-02879-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02879-3