Abstract
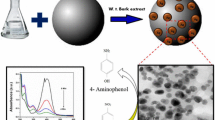
The current study emphases on formulating active hybrid bio-nanocomposite material based on biopolymer alginate embedded with TiO2 and silver nanoparticles. A fixed amount of TiO2 nanodispersion was mixed with alginate solution then the Alginate/TiO2 bio-nanocomposite beads obtained by ionic crosslinking using CaCl2 solution. The prepared Alginate/TiO2 beads in the wet state loaded with Ag+ ions by adsorption technique then the Alginate/TiO2 beads loaded with silver ions reduced to silver nanoparticles (Ag NPs) through irradiation technique. The bio-nanocomposite beads Alginate/TiO2 and Alginate/TiO2–Ag fully studied to assess the nanostructure morphology of embedded TiO2 and Ag nanoparticles: size and shape. The catalytic ability of the prepared nanocomposite beads as a catalyst inspected by studying its ability on the reduction of 2- Nitrophenol (2-NP) to 2-Aminophenol (2-AP) under various factors including initial concentration of 2-nitrophenol, time of reduction process, content of reducing agent and amount of catalyst. It is found that the embedding of Ag nanoparticles has a great impact where the catalytic hydrogenation of 2-NP to 2-AP reached to 98% in case of using Alginate/TiO2–Ag compared with 76% in case of applying hybrid Alginate/TiO2 bio-nanocomposite as a catalyst. Also, the prepared hybrid bio-nanocomposite beads has a high reduction ability for 2-nitrophenol up to 0.05 M concentration. The optimum weight of catalyst was 0.4 g which accomplished nearly 98% reduction percentage.















Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
K. Zhang, J.M. Suh, T.H. Lee et al., Copper oxide—graphene oxide nanocomposite : efficient catalyst for hydrogenation of nitroaromatics in water. Nano Converg. (2019). https://doi.org/10.1186/s40580-019-0176-3
M. Shokouhimehr, S.M. Yek, Palladium nanocatalysts on hydroxyapatite: green oxidation of alcohols and reduction of nitroarenes in water. Appl. Sci. (2019). https://doi.org/10.3390/app9194183
K. Zhang, J.M. Suh, J. Choi et al., Recent advances in the nanocatalyst-assisted NaBH4 reduction of nitroaromatics in water. ACS Omega 4, 483–495 (2029). https://doi.org/10.1021/acsomega.8b03051
F. Anjum, S. Gul, M.I. Khan, M.A. Khan, Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes. Green Process. Synth. 9, 63–76 (2020). https://doi.org/10.1515/gps-2020-0008
M. Zhao, K. Yuan, Y. Wang et al., Metal–organic frameworks as selectivity regulators for hydrogenation reactions. Nat. Publ. Gr. 539, 76–80 (2026). https://doi.org/10.1038/nature19763
H. Liu, X. Liu, Y. Li et al., Hollow PtNi alloy nanospheres with enhanced activity and methanol tolerance for the oxygen reduction reaction. Nano Res. 9(11), 3494–3503 (2016). https://doi.org/10.1007/s12274-016-1226-3
Y. Gu, Y. Jiao, X. Zhou et al., Strongly coupled Ag/TiO2 heterojunctions for effective and stable photothermal catalytic reduction of 4-nitrophenol. Nano Res. 11(1), 126–141 (2018). https://doi.org/10.1007/s12274-017-1612-5
H. Karimi-Maleh, B.G. Kumar, S. Rajendran et al., Tuning of metal oxides photocatalytic performance using Ag nanoparticles integration. J. Mol. Liq. 2020(314), 113588 (2020). https://doi.org/10.1016/j.molliq.2020.113588
M.M. Ba-abbad, A.A.H. Kadhum, Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation. Int. J. Electrochem. Sci. 7, 4871–4888 (2012)
A. Ziashahabi, M. Prato, Z. Dang et al., The effect of silver oxidation on the photocatalytic activity of Ag/ZnO hybrid plasmonic/metal-oxide nanostructures under visible light and in the dark. Sci. Rep. 9, 1–12 (2019). https://doi.org/10.1038/s41598-019-48075-7
S. Aftab, N.K. Bakirhan, O. Esim et al., NH2-fMWCNT-titanium dioxide nanocomposite based electrochemical sensor for the voltammetric assay of antibiotic drug nadifloxacin and its in vitro permeation study. J. Electroanal. Chem. 859, 113857 (2020). https://doi.org/10.1016/j.jelechem.2020.113857
H.M. Alshammari, Synthesis of palladium and copper nanoparticles supported on TiO2 for oxidation solvent-free aerobic oxidation of benzyl alcohol. Processes (2021). https://doi.org/10.3390/pr9091590
S. Moqadam, M. Salami-Kalajahi, M. Mahdavian, Synthesis and characterization of sunflower oil-based polysulfide polymer/cloisite 30B nanocomposites. Iran J. Chem. Chem. Eng. 37, 185–192 (2018)
M.M. Khan, S. Kalathil, J. Lee, M.H. Cho, Enhancement in the photocatalytic activity of Au@TiO2 nanocomposites by pretreatment of TiO2 with UV light. Bull. Korean Chem. Soc. 33, 1753–1758 (2012). https://doi.org/10.5012/bkcs.2012.33.5.1753
N. Sahu, K.M. Parida, Photocatalytic Activity of Au/TiO2 Nanocomposite for Azo dyes degradation. Kinet. Catal. 53(2), 197–205 (2012). https://doi.org/10.1134/S0023158412020097
P. Saikia, A.T. Miah, P.P. Das, Highly efficient catalytic reductive degradation of various organic dyes by Au/CeO2-TiO2nano-hybrid. J. Chem. Sci. 2017(129), 81–93 (2017). https://doi.org/10.1007/s12039-016-1203-0
B.B. Tripathy, M. Behera, H. Rath et al., Evolution of microstructure and optical properties of TiO2/Au nanocomposite. Indian J. Pure Appl. Phys. 57, 95–100 (2019)
M.A. Gondal, S.G. Rashid, M.A. Dastageer et al., Sol-gel synthesis of Au/Cu-TiO2 nanocomposite and their morphological and optical properties. J. IEEE Photon. (2013). https://doi.org/10.1109/JPHOT.2013.2262674
P. Martins, S. Kappert, H.N. Le et al., Enhanced photocatalytic activity of au/TiO2 nanoparticles against ciprofloxacin. Catalysts (2020). https://doi.org/10.3390/catal10020234
A. Biswas, A. Corani, A. Kathiravan et al., Control of the size and shape of TiO2 nanoparticles in restricted media. Nanotechnology (2013). https://doi.org/10.1088/0957-4484/24/19/195601
L. Kaufner, R. Cartier, R. Wüstneck et al., Poly(ethylene oxide)-block-poly(glutamic acid) coated maghemite nanoparticles: In vitro characterization and in vivo behaviour. Nanotechnology (2007). https://doi.org/10.1088/0957-4484/18/11/115710
H. Fissan, S. Ristig, H. Kaminski et al., Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal. Methods 6, 7324–7334 (2014). https://doi.org/10.1039/c4ay01203h
T.G.F. Souza, V.S.T. Ciminelli, N.D.S. Mohallem, A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. (2016). https://doi.org/10.1088/1742-6596/733/1/012039
C. Kästner, A.F. Thünemann, Au nanoparticles decorated on activated coke via a facile preparation for efficient catalytic reduction of nitrophenols and azo dyes. Langmuir 32, 7383–7391 (2016). https://doi.org/10.1021/acs.langmuir.6b01477
G. Shimoga, R.R. Palem, S.H. Lee, S.Y. Kim, Catalytic degradability of p-nitrophenol using ecofriendly silver nanoparticles. Metals (Basel) 10, 1–20 (2020). https://doi.org/10.3390/met10121661
J. Kaur, J. Singh, M. Rawat, An efficient and blistering reduction of 4-nitrophenol by green synthesized silver nanoparticles. SN Appl. Sci. 1, 1–6 (2019). https://doi.org/10.1007/s42452-019-1088-x
Y. Fu, P. Xu, D. Huang et al., Au nanoparticles decorated on activated coke via a facile preparation for efficient catalytic reduction of nitrophenols and azo dyes. Appl. Surf. Sci. 473, 578–588 (2019). https://doi.org/10.1016/j.apsusc.2018.12.207
S. Mehmood, N.K. Janjua, F. Saira, H. Fenniri, AuCu@Pt nanoalloys for catalytic application in reduction of 4-nitrophenol. J. Spectrosc. (2016). https://doi.org/10.1155/2016/6210794
C. Mariia, M. Natalia, Z. Vladimir, S. Mikhail, F.L. Leonarda, M. Grigory, Room-temperature nitrophenol reduction over Ag–CeO2 catalysts: the role of catalyst preparation method. Catalysts 10, 580 (2020). https://doi.org/10.3390/catal10050580
K.G. Vinod, A. Necip, L.Y. Mehmet, U. Zafer, U. Lokman, A novel magnetic Fe@Au coreeshell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res. 48, 210–217 (2014)
G.B. Neus et al., Hollow PdAg-CeO2 heterodimer nanocrystals as highly structured heterogeneous catalysts. Sci. Rep. 9, 18776 (2019). https://doi.org/10.1038/s41598-019-55105-x
S.R. Thawarkar, B. Thombare, B.S. Munde, N.D. Khupse, Kinetic investigation for the catalytic reduction of nitrophenol using ionic liquid stabilized gold. RSC Adv. 8, 38384–38390 (2018). https://doi.org/10.1039/c8ra07404f
J. Balou, M.A. Khalilzadeh, D. Zareyee, An efficient and reusable nano catalyst for the synthesis of benzoxanthene and chromene derivatives. Sci. Rep. 9, 3605 (2019). https://doi.org/10.1038/s41598-019-40431-x
Acknowledgements
The authors extend their appreciation to the Deputyship for research and innovation, Ministry of Education in Saudi Arabia for funding this research work through the Project Number (IF2/PSAU/2022/01/22988)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Faten Abou El Fadl wrote the main manuscript text and Manal F. Abou Talib prepared figures. All authors reviewed the manuscript."
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article do not have any conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fadl, F.I.A.E., Taleb, M.F.A. Hybrid Alginate/TiO2/Ag Bio-nanocomposite Beads for Catalytic Hydrogenation of 2-Nitrophenol. J Inorg Organomet Polym 33, 2142–2153 (2023). https://doi.org/10.1007/s10904-023-02651-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02651-7