Abstract
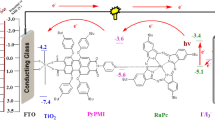
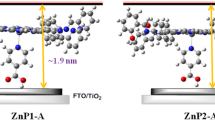
A new self-assemblies based on double-deck dyes ZnTPP-Wi (i = 1–3) were synthesized and applied in dye-sensitized solar cells (DSSCs). Anchoring molecules (Wi i = 1–3) consisting of phenyl carboxyl acid and cyanoacetic acid group. Capping layer dyes zinc meso-tetraphenylporphine (ZnTPP) with anchoring molecules Wi through axially coordination bonds of Zn-to-ligand self-assemblies solar cells devices. We herein report a consisting acylamide and cyanoacetic acid group W3 as an anchoring molecule for the axial coordination with upper zinc porphyrin ZnTPP. W3 was synthesized by introducing acylamide and cyanoacetic acid groups may inhibit adverse dye aggregation and improving electrons are effectively injected into the TiO2 semiconductor surface. Thus, W3 anchoring molecules can be used to fabricate efficient solar cells with ZnTPP porohyrin dye, achieving good photoelectric performance, indicative of their general applicability in fabricating good-performance DSSCs. The assembled modes were also verified by transmission electron microscopy (TEM). The photoelectrochemical efficiencies for dye ZnTPP-W3 are best than those of self-assembly dyes prevailingly ascribed to larger Jsc and Voc.










Similar content being viewed by others
References
F. D’Souza, A.N. Amin, M.E. El-Khouly et al., Control over photoinduced energy and electron transfer in supramolecular polyads of covalently linked azaBODIPY-bisporphyrin “molecular clip” hosting fullerene. J. Am. Chem. Soc. 134, 654–664 (2012)
I. Takahiko, M. Yutaka, N. Eiichi, Photostability of a dyad of magnesium porphyrin and fullerene and its application to photocurrent conversion. Chem. Commun. 49, 279–281 (2013)
H. Zhou, L. Yang, A.C. Stuart et al., Development of fluorinated benzothiadiazole as a structural unit for a polymer solar cell of 7 % efficiency. Angew. Chem. Int. Ed. 50, 2995–2998 (2011)
F.C. Krebs, T. Thomas, J. Mikkel, Upscaling of polymer solar cell fabrication using full roll-to-roll processing. Nanoscale 2, 873–886 (2010)
K. Yu, J. Chen, Enhancing solar cell efficiencies through 1-D nanostructures. Nanoscale Res. Lett. 4, 1–10 (2009)
G. Su, Q. Li, M. Ishida et al., N-confused phlorin-prodigiosin Chimera: meso-aryl oxidation and π-extension triggered by peripheral coordination. Angew. Chem. Int. Ed. 59(4), 1537–1541 (2020)
N. Zarrabi, S. Seetharaman, S. Chaudhuri et al., Decelerating charge recombination using fluorinated porphyrins in N, N-bis (3, 4, 5-trimethoxyphenyl) aniline-Aluminum (III) porphyrin-fullerene reaction center models. J. Am. Chem. Soc. 142(22), 10008–10024 (2020)
N. Jiang, Y. Wang, A. Qin et al., Effective enhancement of the emission efficiency of tetraphenylporphyrin in solid state by tetraphenylethene modification. Chin. Chem. Lett. 30(1), 143–148 (2019)
W. Miao, Z. Zhu, Z. Li et al., Novel expanded porphyrinoids with multiple-inner-ring-fusion and/or tunable aromaticity. Chin. Chem. Lett. 30(11), 1895–1902 (2019)
Y. Tang, X. Liu, Y. Wang et al., Solar cells sensitized by porphyrin dyes containing a substituted carbazole donor with synergistically extended absorption and suppressed the dye aggregation. Chin. Chem. Lett. 31(7), 1927–1930 (2020)
Y. Hu, W.A. Webre, M.B. Thomas et al., β-Functionalized push–pull opp-dibenzoporphyrins as sensitizers for dye-sensitized solar cells: the role of the phenylethynyl bridge. J. Mater. Chem. A 7(17), 10712–10722 (2019)
Y. Chen, K. Zeng, C. Li et al., A new type of multibenzyloxy-wrapped porphyrin sensitizers for developing efficient dye-sensitized solar cells. J. Porphyrins Phthalocya. 24, 401–409 (2020)
Y. Lu, Y. Cheng, C. Li et al., Efficient solar cells based on cosensitizing porphyrin dyes containing a wrapped donor, a wrapped π-framework and a substituted benzothiadiazole unit. Sci. China Chem. 62(8), 994–1000 (2019)
K. Zeng, Y. Lu, W. Tang et al., Efficient solar cells sensitized by a promising new type of porphyrin: dye-aggregation suppressed by double strapping. Chem. Sci. 10(7), 2186–2192 (2019)
C.W. Lee, H.P. Lu, C.M. Lan et al., Novel zinc porphyrin sensitizers for dye-sensitized solar cells: synthesis and spectral, electrochemical, and photovoltaic properties. Chem.-Eur. J. 15(6), 1403–1412 (2009)
A. Hagfeldt, G. Boschloo, L. Sun et al., Dye-sensitized solar cells. Chem. Rev. 110(11), 6595–6663 (2010)
T. Wei, X. Sun, X. Li et al., Systematic investigations on the roles of the electron acceptor and neighboring ethynylene moiety in porphyrins for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 7(39), 21956–21965 (2015)
F. Gou, X. Jiang, R. Fang et al., Strategy to improve photovoltaic performance of DSSC sensitized by zinc prophyrin using salicylic acid as a tridentate anchoring group. ACS Appl. Mater. Interfaces 6(9), 6697–6703 (2014)
F. D’Souza, G.R. Deviprasad, M.E. Zandler et al., Spectroscopic, electrochemical, and photochemical studies of self-assembled via axial coordination zinc porphyrin- fulleropyrrolidine dyads. J. Phys. Chem. A 106(13), 3243–3252 (2002)
T. Honda, T. Nakanishi, K. Ohkubo et al., Formation of a long-lived photoinduced electron-transfer state in an electron acceptor-donor-acceptor porphyrin triad connected by coordination bonds. J. Phys. Chem. C 114(33), 14290–14299 (2010)
J. Otsuki, M. Takatsuki, M. Kaneko et al., Formation of a supramolecular porphyrin-spacer-acceptor ternary complex and intracomplex electron transfer. J. Phys. Chem. A 107(3), 379–385 (2003)
N.K. Subbaiyan, C.A. Wijesinghe, F. D’Souza, Supramolecular solar cells: surface modification of nanocrytalline TiO2 with coordinating ligands to immobilize sensitizers and dyads via metal-ligand coordination for enhanced photocurrent generation. J. Am. Chem. Soc. 131(41), 14646–14647 (2009)
Y. Wu, Q. Zhang, J.C. Liu et al., A novel self-assembly with two acetohydrazide zinc porphyrins coordination polymer for supramolecular solar cells. Org. Electron. 41, 301–306 (2017)
J.X. Zhang, F.M. Han, J.C. Liu et al., Self-assemblies formed by isonicotinic acid analogues axially coordinating with zinc porphyrin via pyridyl unit: synthesis and application in dye sensitized solar cells. Tetrahedron Lett. 57(17), 1867–1872 (2016)
Y. Wu, J.C. Liu, J. Cao et al., Two self-assemblies of Schiff base porphyrins to modify titanium dioxide electrodes for supramolecular solar cells. Res. Chem. Intermed. 41, 6833–6842 (2015)
K.M. Wang, Y.C. Qin, G.J. Cheng et al., Design, synthesis and antibacterial evaluation of novel fluoroquinolone and its derivatives. Asian J. Chem. 26(1), 209–215 (2014)
P. Wang, S.M. Zakeeruddin, P. Comte et al., Enhance the performance of dye-sensitized solar cells by co-grafting amphiphilic sensitizer and hexadecylmalonic acid on TiO2 nanocrystals. J. Phys. Chem. B 107, 14336–14341 (2003)
J.M. Ji, H. Zhou, H.K. Kim, Rational design criteria for D-π-A structured organic and porphyrin sensitizers for highly efficient dye-sensitized solar cells. J. Mater. Chem. A 6(30), 14518–14545 (2018)
B. Chen, L. Sun, Y.S. Xie, Modulation of photovoltaic behavior of dye-sensitized solar cells by electron donors of porphyrin dyes and cosensitization. Chin. Chem. Lett. 26(7), 899–904 (2015)
M. Urbani, M. Grätzel, M.K. Nazeeruddin et al., Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 114(24), 12330–12396 (2014)
M.J. Frisch, G.W. Trucks, H.B. Schlegel et al., Gaussian 09, Revision a. 02, Gaussian, Inc., Wallingford, CT, USA (2009)
P.J. Hay, W.R. Wadt, Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82(1), 270–283 (1985)
W. Lee, S.B. Yuk, J. Choi et al., The effects of the number of anchoring groups and N-substitution on the performance of phenoxazine dyes in dye-sensitized solar cells. Dyes Pigm. 102, 13–21 (2014)
L. Yu, W. Shi, L. Lin et al., Effects of benzo-annelation of asymmetric phthalocyanine on the photovoltaic performance of dye-sensitized solar cells. Dalton Trans. 43(22), 8421–8430 (2014)
S. Soman, M.A. Rahim, S. Lingamoorthy et al., Strategies for optimizing the performance of carbazole thiophene appended unsymmetrical squaraine dyes for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 17(35), 23095–23103 (2015)
A. Kira, T. Umeyama, Y. Matano et al., Supramolecular donor-acceptor heterojunctions by vectorial stepwise assembly of Porphyrins and coordination-bonded fullerene arrays for photocurrent generation. J. Am. Chem. Soc. 131(9), 3198–3200 (2009)
C. Yang, Z. Yang, H. Gu et al., Facet-selective 2D self-assembly of TiO2 nanoleaves via supramolecular interactions. Chem. Mater. 20(24), 7514–7520 (2008)
J. Cao, J.C. Liu, W.T. Deng et al., A novel self-assembly with zinc porphyrin coordination polymer for enhanced photocurrent conversion in supramolecular solar cells. Electrochim. Acta 112, 515–521 (2013)
H. Choi, Y.S. Chen, K.G. Stamplecoskie et al., Boosting the photovoltage of dye-sensitized solar cells with thiolated gold nanoclusters. J. Phys. Chem. Lett. 6(1), 217–223 (2015)
Z.S. Wang, Y. Cui, K. Hara et al., A high-light-harvesting-efficiency coumarin dye for stable dye-sensitized solar cells. Adv. Mater. 19(8), 1138–1141 (2007)
A.O. Biroli, F. Tessore, V. Vece et al., Highly improved performance of Zn II tetraarylporphyrinates in DSSCs by the presence of octyloxy chains in the aryl rings. J. Mater. Chem. A 3(6), 2954–2959 (2015)
C. Chen, X. Yang, M. Cheng et al., Efficient panchromatic organic sensitizers with dihydrothiazole derivative as π-bridge for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 5(21), 10960–10965 (2013)
S. Bok Joo, S. Hae Min, C. In Taek et al., A desirable hole-conducting coadsorbent for highly efficient dye-sensitized solar cells through an organic redox cascade strategy. Chem. A Eur. J. 17, 11115–11121 (2011)
H.M. Song, D.S. Kang, S.K. Min et al., A simple triaryl amine-based dual functioned co-adsorbent for highly efficient dye-sensitized solar cells. J. Mater. Chem. 22, 3786–3794 (2012)
W. Zhang, Y. Wu, H. Zhu et al., Rational molecular engineering of indoline-based D-A-π-A organic sensitizers for long-wavelength-responsive dye-sensitized solar cells. ACS Appl. Mater. Interfaces 7(48), 26802–26810 (2015)
J.M. Andrés-Castán, S. Franco, B. Villacampa et al., New efficient tert-butyldiphenyl-4 H-pyranylidene sensitizers for DSSCs. RSC Adv. 5(129), 106706–106709 (2015)
Acknowledgements
The National Natural Science Foundation of China (No. 21461023); Guizhou Province Science Foundation (QianKeHe-ZK[2021]064); Natural Science Foundation of Guizhou Provincial Department of Education (QianJiaoHe KY Zi [2020]200; [2020]203) and Major projects of research and Innovation Fund of Qiannan Normal University for Nationalities (No. QNSY2018BS020) have supported this work. We are very grateful to Prof. Peng Wang (Changchun Institute of Applied Chemistry, Chinese Academy of Sciences) for supplying device fabrication and measurement of solar cells. We also acknowledge the support of Key Laboratory of Computational Catalytic Chemistry of Guizhou Province. The authors also thank Shiyanjia Lab (www.shiyanjia.com) for the HRMS and 1H NMR measurement and analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Y., Wang, QM., Wang, F. et al. Novel Self-assembled Isonicotinic Acid Derivative and Zinc Porphyrin Dyads and Applications in Dye Sensitized Solar Cells. J Inorg Organomet Polym 32, 3196–3203 (2022). https://doi.org/10.1007/s10904-022-02332-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02332-x