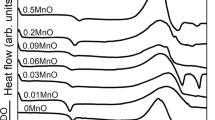
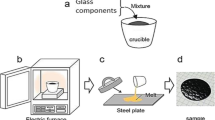
Abstract
Phosphate glasses containing different concentrations of zinc oxide inside the (1 − x)(NaPO3–KPO3)–xZnO system (0 ≤ x ≤ 50 mol%) have been prepared using the conventional melt quenching technique. The prepared glasses were transparent, bubble-free and colourless. Their density, molar volume, glass transition temperature, and structural properties using infrared and Raman spectroscopies are investigated. As the content of ZnO increases, the density increases while the molar volume decreases. The composition dependence of Tg shows a minimum for the glass (x = 20 mol%). Structural approach realized by IR and Raman spectroscopies reveals that zinc ions occupy different sites in the glassy-network, mainly modifier sites and middle phosphate network in low-zinc and high-zinc glasses, respectively. The introduction of ZnO in the network induces some structural rearrangements through the conversion of metaphosphate structural units to pyrophosphate ones. It is also highlighted that the presence of ZnO in the glassy matrix allows the transformation of some P–O–P and P=O bonds to P–O–Zn linkages. From the UV–Visible absorption studies, the values of the optical band gap, Eg, and Urbach energy, ΔE, were evaluated. The optical band gap is found to depend on the glasses composition. Eg and ΔE show a minimum and a maximum respectively, for the glass (x = 20 mol%).










Similar content being viewed by others
References
B. Tischendorf, J.U. Otaigbe, J.W. Wiench, M. Pruski, B.C. Sales, A study of short and intermediate range order in zinc phosphate glasses. J. Non Cryst. Solids 282(2–3), 147–158 (2001)
R.V.S.S.N. Ravikumar, K. Ikeda, A.V. Chandrasekhar, Y.P. Reddy, P.S. Rao, J. Yamauchi, Site symmetry of Mn(II) and Co(II) in zinc phosphate glass. J. Phys. Chem. Solids 64(12), 2433–2436 (2003)
G. Walter, U. Hoppe, J. Vogel, G. Carl, P. Hartmann, The structure of zinc polyphosphate glass studied by diffraction methods and31P NMR. J. Non Cryst. Solids 356(43), 252–262 (2010)
P. Pascuta, G. Borodi, N. Jumate, I. Vida-Simiti, D. Viorel, E. Culea, The structural role of manganese ions in some zinc phosphate glasses and glass ceramics. J. Alloys Compd. 504(2), 479–483 (2010)
E. Mansour, G. El-Damrawi, Electrical properties and FTIR spectra of ZnO-PbO-P2O5 glasses. Phys. B Condens. Matter. 405(8), 2137–2143 (2010)
J.J. Hudgens, R.K. Brow, D.R. Tallant, S.W. Martin, Raman spectroscopy study of the structure of lithium and sodium ultraphosphate glasses. J. Non Cryst. Solids 223(1–2), 21–31 (1998)
P.Y. Shih, S.W. Yung, T.S. Chin, Thermal and corrosion behavior of P2O5-Na2O-CuO glasses. J. Non Cryst. Solids 224(2), 143–152 (1998)
R. Morena, Phosphate glasses as alternatives to Pb-based sealing frits. J. Non-Cryst. Solids 264, 382–387 (2000)
G. Tricot, B. Revel, S. Wegner, Thermal stability of a low Tg phosphate glass investigated by DSC, XRD and solid state NMR. J. Non Cryst. Solids 357(14), 2708–2712 (2011)
J. Cha, T. Kubo, H. Takebe, M. Kuwabara, Compositional dependence of properties of SnO-P2O5 glasses. J. Ceram. Soc. Japan 116, 915–919 (2008)
R. Oueslati, S. Krimi, J. Jacques, I. Khattech, A. El, M. Jemal, Structural and thermochemical study of Na2O–ZnO–P2O5 glasses. J. Non-Cryst. Solids 390, 5–12 (2014)
G.P. Singh, S. Kaur, P. Kaur, D.P. Singh, Modification in structural and optical properties of ZnO, CeO2 doped Al2O3PbOB2O3 glasses. Phys. B Condens. Matter 407(8), 1250–1255 (2012)
M.H.M. Zaid et al., Investigation on structural and optical properties of SLS–ZnO glasses prepared using a conventional melt quenching technique. J. Mater. Sci.: Mater. Electron. 26(6), 3722–3729 (2015)
W.H. Zachariasen, Atomic arrangement in glass. Jour. Amer. Chem. Soc. 54(10) 3841–3851 (1932)
M.S. Reddy, G.M. Krishna, N. Veeraiah, Spectroscopic and magnetic studies of manganese ions in ZnO-Sb2O3-B2O3 glass system. J. Phys. Chem. Solids 67(4), 789–795 (2006)
W. Matz, D. Stachel, The structure of alkaline earth metaphosphate glasses investigated by neutron diffraction. J. Non-Cryst. Solids 101, 80–89 (1988)
U. Cadi, A. Marrakech, En cotutelle et Spécialité: Physico-chimie de la Matière Condensée (2003)
R.K. Brow, D.R. Tallant, S.T. Myers, C.C. Phifer, The short-range structure of zinc polyphosphate glass. J. Non Cryst. Solids 191(1–2), 45–55 (1995)
L. Montagne, G. Palavit, R. Delaval, Effect of ZnO on the properties of (100–x)(NaPO3)-xZnO glasses. J. Non Cryst. Solids 223, 43–47 (1998)
Y. Zhang, Ionocovalency and applications 3. Ionocovalent crosslink density, pp. 1–6 (2011)
Y. Zhang, Ionocovalency and applications 1. Ionocovalency model and orbital hybrid scales, pp. 4381–4406 (2010)
M. El Hezzat, M. Et-tabirou, L. Montagne, E. Bekaert, G. Palavit, Structure and ac conductivity of sodium–lead–cadmium metaphosphate glasses. Mater. Lett. 58, 60–66 (2003)
I. Konidakis, C.P.E. Varsamis, E.I. Kamitsos, D. Möncke, D. Ehrt, Structure and properties of mixed strontium-manganese metaphosphate glasses. J. Phys. Chem. C 114(19), 9125–9138 (2010)
S.S. Das, V. Srivastava, Study of sodium and silver phosphate glasses doped with some metal chlorides. Prog. Cryst. Growth Charact. Mater. 52(1–2), 125–131 (2006)
A.S. Budi, A.H. Permana, H. Nasbey, Y. Fujii, The characterization ZnO-Na2O-P2O5 glass system for dental restorative materials. J. Tech. Soc. Sci. 1(3), 47–55 (2017)
B.Ã. Eraiah, S.G. Bhat, Optical properties of samarium doped zinc–phosphate glasses. J. Phys. Chem. Solids 68, 581–585 (2007)
M.A. Ghauri, S.A. Siddiqi, W.A. Shah, M.G.B. Ashiq, M. Iqbal, Optical properties of zinc molybdenum phosphate glasses. J. Non Cryst. Solids 355(50–51), 2466–2471 (2009)
H. Es-sou, L. Bih, B. Manoun, P. Lazor, Structure, thermal analysis and optical properties of lithium tungsten-titanophosphate glasses. J. Non-Cryst. Solids 463, 12–18 (2017)
E.A. Davis, N.F. Mott, Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Phil. Mag. 22(179), 903–922 (1970)
F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 92, 61 (1953)
N.H. Ray, Composition-property relationships in inorganic oxide glasses. J. Non-Cryst. Solids 15(3), 423–434 (1974)
H. Bih et al., Thermal and structural studies of Li2O-Na2O-SrO-TiO2-B2O3-P2O5 glasses by DTA, IR and EPR spectroscopy. J. Appl. Surf. Interfaces 1, 57–63 (2017)
C. A. Gutierrez, Study of a mixed alkaline–earth effect on some properties of the CaO-MgO-Al2O3-SiO2 system, no. May 2014 (2007)
F. Behrends, H. Eckert, Mixed-alkali effects in aluminophosphate glasses: a re-examination. J. Phys. Chem. C 3, 17175–17183 (2011)
A.L. Allred, Electronegativity values from thermochemical data. J. Inorg. Nuclear Chem. 17(1949), 215–221 (1961)
L. Bih, N. Allali, A. Yacoubi, A. Nadiri, A. Levasseur, Thermal, physical and spectroscopic investigations of P2O5—A2MoO4–A2O (A = Li, Na) glasses. Phys. Chem. Glasses 40(4), 229–234 (1999)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A32, 751–767 (1976)
A. Chahine, M. Et-Tabirou, M. Elbenaissi, M. Haddad, J.L. Pascal, Effect of CuO on the structure and properties of (50–x/2)Na2O-xCuO-(50–x/2)P2O5 glasses. Mater. Chem. Phys. 84(2–3), 341–347 (2004)
J.O. Byun, B.H. Kim, K.S. Hong, H.J. Jung, S.W. Lee, A.A. Izyneev, Properties and structure of RO-Na2O-Al2O3-P2O5(R = Mg, Ca, Sr, Ba) glasses. J. Non Cryst. Solids 190(3), 288–295 (1995)
B. Bae, M.C. Weinberg, Ultraviolet optical absorptions of semiconducting copper phosphate glasses. Ultraviolet glasses optical absorptions of semiconducting copper phosphate. J. Appl. Phys. 7760(1993), 0–7 (2014)
S.F. Khor, Z.A. Talib, W.M.M. Yunus, Optical properties of ternary zinc magnesium phosphate glasses. Ceram. Int. 38(2), 935–940 (2012)
G. Srinivas, J.S. Kumar, M.N. Chary, R. Sayanna, EPR and optical studies on binary mixed alkali alkaline earth oxide borate glasses doped with Cu2+ ions. Glass Phys. Chem. 42(2), 141–148 (2016)
Acknowledgements
The authors would like to thank the CNRST (Morocco) and OCP foundation for their financial support of this work in the framework of PPR project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jerroudi, M., Bih, L., Azrour, M. et al. Investigation of Novel Low Melting Phosphate Glasses Inside the Na2O–K2O–ZnO–P2O5 System. J Inorg Organomet Polym 30, 532–542 (2020). https://doi.org/10.1007/s10904-019-01213-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01213-0