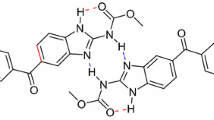
Abstract
A novel series, of thioacetanilide derivatives (4a–e), was synthesized and fully characterized. Their corresponding VO(II) complexes, were prepared and inspected by all analytical, spectral and computational techniques. From IR spectral analysis, poly dentate mode of bonding, was proposed for all organic ligands towards two central atoms. The octahedral arrangement configuration, was suggested for all complexes, based on; UV–Vis, ESR and magnetic measurements. TGA and DTA analysis, built a good assertion on the presence of solvent molecules, attached with the complex particles. The molecular modeling technique, excreted the best structural forms for all tested compounds. Moreover, essential computational parameters were estimated, to verify the molecular formulae. Molecular docking study, was concerning with the inhibition feature against 3s7s and 3gcw proteins, belonging to breast and liver cancer cells, respectively. The computed parameters introduced a promising activity for some tested derivatives. Furthermore, the experimental antitumor screening for VO(II) complexes, versus HepG-2 and MCF-7 cell lines, displayed superiority for [(VO)2(SO4)2(AETA)(H2O)2]3H2O complex in comparing to cisplatin (standard drug). As well as, the genotoxicity results, coincide excellently with antitumor result.










Similar content being viewed by others
References
K. Savithri, H.D. Revanasiddappa, Bioinorg. Chem. Appl. 2018, 1 (2018)
J. Korbecki, I. Baranowska-Bosiacka, I. Gutowska, D. Chlubek, Acta Biochim. Polonica 59, 195 (2012)
I.M. El-Deen, A.F. Shoair, M.A. El-Bindary, J. Mol. Stru. 1180, 420–437 (2019)
E. Bagdatli, E. Altuntas, U. Sayin, J. Mol. Stru. 1127, 653 (2017)
J. Wei, J. Xiao, J. Yu, X. Yi, S. Liu, G. Liu, Polyhedron 137, 176 (2017)
I. Althagafi, N.M. El-Metwally, M. Elghalban, T.A. Farghaly, A.M. Khedr, Bioinorg. Chem. Appl. 2018, 1 (2018)
G. Bao, B. Du, Y. Ma, M. Zhao, P. Gong, X. Zhai, Med. Chem. 12, 489 (2016)
Q.P. Peterson, D.C. Hsu, D.R. Goode, C.J. Novotny, R.K. Totten, P.J. Hergenrother, J. Med. Chem. 52, 5721 (2009)
H.M.A. Abumelha, J. Heterocycl. Chem. 55, 1738 (2018)
D. Osmaniye, S. Levent, A.B. Karaduman, S. Ilgın, Y. Özkay, Z.A. Kaplancıkl, Molecules 23, 1 (2018)
M.T. Gabr, N.S. El-Gohary, E.R. El-Bendary, M.M. El-Kerdawy, Eur. J. Med. Chem. 85, 576 (2014)
S. Farkona, E.P. Diamandis, I.M. Blasutig, BMC Med. 14, 73 (2016)
A.H. Harhash, F.A. Amer, M.A.N. Eldin, M.L. Awad, Z. Naturforsch. 31B, 846 (1976)
M.E. Reichmann, C.A. Rice, C.A. Thomos, P. Doty, J. Am. Chem. Soc. 76, 3047 (1954)
A. Wolfe, G.H. Shimer, T. Meehan, Biochemistry 26, 6392 (1987)
N.M. El-Metwaly, M.S. Refat, Spectrochim. Acta Part A 78, 196–204 (2011)
T. Mosmann, J. Immunol. Methods 65, 55 (1983)
S.M. Gomha, S.M. Riyadh, E.M. Mahmmoud, M.M. Elaasser, Chem. Heterocycl. Compd. 51, 1030 (2015)
A.I. Vogel, Text Book of Quantitative Inorganic Analysis (Longman, London, 1986)
G.A. Bain, J.F. Berry, Chem. Educ. 85, 532 (2008)
E.S. Freeman, B. Carroll, J. Phys. Chem. 62, 394 (1958)
W. Coats, J.P. Redfern, Nature 201, 68 (1964)
T. Ozawa, Bull. Chem. Sot. Japan. 38, 1881 (1965)
W.W. Wendlandt, Thermal Methods of Analysis (Wiley, New York, 1974)
J.H.F. Flynn, L.A. Wall, J. Res. Natl. Bur. Stand. A. 70, 487 (1996)
P. Kofstad, Nature 179, 1362 (1957)
H.W. Horowitz, G.A. Metzger, Anal. Chem. 35, 1464 (1963)
X. Wu. A.K. Ray, Phys. Rev. B 65, 85403 (2002)
M.J. Frisch et al., Gaussian 09, Revision D (Gaussian, Inc., Wallingford, CT, 2010)
R. Dennington, T. Keith, J. Millam. Gauss View, Version 4.1.2, Semichem Inc, Shawnee Mission, KS (2007)
U. El-Ayaan, N.M. El-Metwally, M.M. .Youssef, S.A.A. El Bialy, Spectrochim. Acta A 68, 1278 (2007)
R.K. Ray, G.R. Kauffman, Inorg. Chem. Acta 173, 207 (1990)
M.M. Al-Iede, J. Karpelowsky, D.A. Fitzgerald, Pediatr. Pulmonol. 51, 394 (2016)
T.A. Halgren, J. Comput. Chem. 17, 490 (1998)
G.M. Morris, D.S. Goodsell, R.S. Halliday, R. Huey, W.E. Hart, R.K. Belew, A.J. Olson, J. Comput. Chem. 19, 1639 (1998)
D.S. Solis, R.J.B. Wets, Math. Oper. Res. 6, 19 (1981)
W.J. Geary, Coord. Chem. Rev. 7, 81 (1971)
N.M. El-Metwaly, R.M. El-shazly, I.M. Gabr, A.A. El-Asmy, Spectrochim. Acta A 61, 1113 (2005)
K.S. Abu-Melhaa, N.M. El-Metwally, Spectrochim. Acta A 70, 277 (2008)
K. Nakamoto, Infrared Raman Spectra of Inorganic and Coordination Compounds (Wiley–Interscience, New York, 1986)
A.A. Abou-Hussen, N.M. El-Metwaly, E.M. Saad, A.A. El-Asmy, Coord. Chem. 58, 1735 (2005)
A.B.P. Lever, Inorganic Electronic Spectroscopy (Elsevier, Amsterdam, 1986)
H. Yokoi, A.W. Addison, Inorg. Chem. 16, 1341 (1977)
B.J. Hathaway, D.E. Billing, Coord. Chem. Rev. 5, 143 (1970)
T.M. Dunn, Trans. Faraday Soc. 57, 1441 (1961)
R.C. Chikate, S.B. padhye, Polyhedron 24, 1689 (2005)
B.D. Cullity, Elements of X-Ray Diffraction (Addison-Wesley Publishing Company Inc., Phillippines, 1978)
S. Velumani, X. Mathew, P.J. Sebastian, S.K. Narayandass, D. Mangalaraj, Sol. Cells 76, 347 (2003)
J.S. Ritch, T. Chivers, K. Ahmad, M. Afzaal, P.O. Brien, Inorg. Chem. 49, 1198 (2010)
A.Z. El-Sonbati, A.F. Shoair, A.A. El-Bindary, M.A. Diab, A.S. Mohamed, J. Mol. Liq. 209, 635 (2015)
L.H. Abdel-Rahman, A.M. Abu-Dief, N.M. Ismail, M. Ismael, J. Inorg, Nano-Metal Chem. 47, 467 (2017)
R. Sabet, M. Mohammadpour, A. Sadeghi, A. Fassihi, Eur. J. Med. Chem. 45, 1113 (2010)
M. Kumar, K. Ramasamy, V. Mani, R.K. Mishra, A.B.A. Majeed, E. De Clercq, B. Narasimhan, Arab. J. Chem. 7, 396 (2014)
I. Fleming, Frontier Orbital’s and Organic Chemical Reactions (Wiley, London, 1976)
S.K. Tripathi, R. Muttineni, S.K. Singh, J. Theor. Biol. 334, 87 (2013)
F.A. Saad, J.H. Al-Fahemi, H. El-Ghamry, A.M. Khedr, M.G. Elghalban, N.M. El-Metwaly, J. Therm. Anal. Calorim. 131, 1249 (2018)
N. Terakado, S. Shintani, Y. Nakahara, Oncol. Rep. 7, 1113 (2000)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saad, F., El-Metwaly, N. & Khedr, A.M. Synthesis, Characterization for New Nanometric VO(II)–Thioacetanilide Complexes by, Spectral, Thermal, Molecular Computations and DNA Interaction Study Beside Promising Antitumor Activity. J Inorg Organomet Polym 29, 1606–1624 (2019). https://doi.org/10.1007/s10904-019-01124-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01124-0