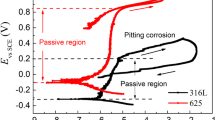
Abstract
Electrochemical techniques were used to investigate different factors such as concentration, immersion time, and temperature on the corrosion behavior of the metal–metal glassy Fe78Co9Cr10Mo2Al1 (VX9) and Fe49Co49V2 (VX50) (at.%) alloys in sulfuric acid (H2SO4). The passive film on the surface of the alloys at different H2SO4 concentrations was analyzed by X-ray photoelectron spectroscopy, and examined by scanning electron microscope and atomic force microscopy. The electrochemical measurements illustrate that the VX9 alloy has a lower corrosion resistivity than the VX50 alloy in all measured effects in this study, due to the n-type semiconductor passive layer (FeOOH, Fe3O4, and Fe2O3) that forms on this alloy. This occurred even though these alloys demonstrated a high degree of resistance at higher concentrations (12.0 M) of H2SO4 solution. A decrease in the corrosion resistance and an increase in the corrosion rate was observed with the incremental increase of immersion time and temperature. The thermodynamic parameters, such as \({\text{E}}_{{\text{a}}}^{*}\), ΔS* and ΔH*, were evaluated for the corrosion process and discussed in terms of a 3.0 M H2SO4 solution.






Similar content being viewed by others
References
R. Babilas, R. Nowosielski, Iron-based bulk amorphous alloys. Arch. Mater. Sci. Eng. 44, 5–27 (2010)
W.H. Wang, C. Dong, C.H. Shek, Bulk metallic glasses. Mater. Sci. Eng. R 44, 45–89 (2004)
C. Suryanarayana, A. Inoue, Bulk Metallic Glasses (CRC Press, Boca Raton, 2011)
H.F. Li, Y.F. Zheng, Recent advances in bulk metallic glasses for biomedical applications. Acta Biomater. 36, 1–20 (2016)
A. Inoue, B.L. Shen, C.T. Chang, Fe- and Co-based bulk glassy alloys with ultrahigh strength of over 4000 MPa. Intermetallics 14, 936–944 (2006)
Y. Li, X. Jia, W. Zhang, C. Fang, X. Wang, F. Qin, S. Yamaura, Y. Yokoyama, Effects of alloying elements on the thermal stability and corrosion resistance of an Fe-based metallic glass with low glass transition temperature. Metall. Mater. Trans. A 45, 2393–2398 (2014). https://doi.org/10.1007/s11661-013-2071-6
H.B. Fan, W. Zheng, G.Y. Wang, P.K. Liaw, J. Shen, Corrosion behavior of Fe41Co7Cr15Mo14C15B6Y2 bulk metallic glass in sulfuric acid solutions. Metall. Mater. Trans. A 42, 524–533 (2011). https://doi.org/10.1007/s11661-010-0500-3
J.J. Si, X.H. Chen, Y.H. Cai, Y.D. Wu, T. Wang, X.H. Hui, Corrosion behavior of Cr-based bulk metallic glasses in hydrochloric acid solutions. Corros. Sci. 107, 123–132 (2016)
Z. Deng, X.H. Zhang, K.C. Chan, L. Liu, T. Li, Fe-based metallic glass catalyst with nanoporous surface for azo dye degradation. Chemosphere 174, 76–81 (2017)
A.K. Al-Harbi, K.M. Emran, Effect of immersion time on electrochemical and morphology of new Fe-Co metal-metal glassy alloys in acid rain. Arab. J. Chem. (2018). https://doi.org/10.1016/j.arabjc.2018.01.019
K.M. Emran, H. AL-Refai, Pitting resistivity of Ni-based bulk metallic glasses in chloride solution. RSC Adv. 7, 37349–37358 (2017)
K.M. Emran, H. AL-Refai, Electrochemical and surface investigation of Ni-Cr glassy alloys in nitric acid solution. Int. J. Electrochem. Sci. 12, 6404–6416 (2017)
K.M. Emran, A.K. Al-Harbi, Outstanding resistance and passivation behaviour of new Fe-Co metal-metal glassy alloys in alkaline media. PLoS ONE (2018). https://doi.org/10.1371/journal.pone.0187567
K.M. Emran, H. Al-refai, Immersion time effects on the corrosion and passivation characterization of Ni–Cr glassy alloys in artificial seawater. Desalin. Water Treat. 102, 165–172 (2018). https://doi.org/10.5004/dwt.2018.21828
R.S. Gonçalves, D.S. Azambuja, A.M.S. Lucho, Electrochemical studies of propargyl alcohol as corrosion inhibitor for nickel, copper, and copper/nickel (55/45) alloy. Corros. Sci. 44, 467–479 (2002)
Z. Tao, S. Zhang, W. Li, B. Hou, Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros. Sci. 51, 2588–2595 (2009)
R. Amini, H. Vakili, B. Ramezanzadeh, Studying the effects of poly (vinyl) alcohol on the morphology and anti-corrosion performance of phosphate coating applied on steel surface. J. Taiwan Inst. Chem. Eng. 58, 542–551 (2016)
S.S. Abdel Rehim, H.H. Hassan, M.A. Amin, Corrosion and corrosion inhibition of Al and some alloys in sulphate solutions containing halide ions investigated by an impedance technique. Appl. Surf. Sci. 187, 279–290 (2002)
M. Anik, G. Celikten, Analysis of the electrochemical reaction behavior of alloy AZ91 by EIS technique in H3PO4/KOH buffered K2SO4 solutions. Corros. Sci. 49, 1878–94 (2007)
G. Baril, C. Blanc, N. Pébère, Influence of microstructure on the corrosion behavior of two magnesium alloys by EIS, in: Corrosion and Corrosion Prevention of Low Density Metals and Alloys: Proceedings of the International Symposium, vol. 23, ed. by B.A. Shaw, R.G. Buchheit, J.P. Moran (The Electrochemical Society, New Jersey, 2001), pp. 166–175
R. Pinto, M.G.S. Ferreira, M.J. Carmezim, M.F. Montemor, The corrosion behaviour of rare-earth containing magnesium alloys in borate buffer solution. Electrochim. Acta. 56, 1535–1545 (2011)
R. Arrabal, A. Pardo, M.C. Merino, M. Mohedano, P. Casajús, K. Paucar, G. Garcés, Effect of Nd on the corrosion behaviour of AM50 and AZ91D magnesium alloys in 3.5 wt.% NaCl solution. Corros. Sci. 55, 301–312 (2012). https://doi.org/10.1016/j.corsci.2011.10.033
S.T. Arab, K.M. Emran, H.A. Al-Turaif, Passivity characteristics on Ni(Cr)(Fe)SiB glassy alloys in phosphate solution. J. Saudi Chem. Soc. 18, 169–182 (2014). https://doi.org/10.1016/j.jscs.2011.05.020
A.A. El Miligy, D. Geana, W.J. Lorenz, A theoretical treatment of the kinetics of iron dissolution and passivation. Electrochim. Acta 20, 273–281 (1975). https://doi.org/10.1016/0013-4686(75)90005-5
P. Lorbeer, W.J. Lorenz, The kinetics of iron dissolution and passivation depending on temperature and ionic strength. Corros. Sci. 20, 405–412 (1980). https://doi.org/10.1016/0010-938X(80)90008-6
P. Lorreer, W.J. Lorenz, The kinetics of iron dissolution and passivation in solutions containing oxygen. Electrochim. Acta 27, 375–381 (1980). https://doi.org/10.1016/0013-4686(80)87026-5
E. Kikuti, R. Conrrado, N. Bocchi, S.R. Biaggio, R.C. Rocha-Filho, Chemical and electrochemical coloration of stainless steel and pitting corrosion resistance studies. J. Braz. Chem. Soc. 15, 472–480 (2004). https://doi.org/10.1590/S0103-50532004000400005
A. Pardo, E. Otero, M.C. Merino, López,; M.D., M.V. Utrilla, F. Moreno, Influence of pH and chloride concentration on the pitting and crevice corrosion behavior of high-alloy stainless steels. Corrosion 56, 411–418 (2000). https://doi.org/10.5006/1.3280545
A.I. Muñoz, J.G. Antón, J.L. Guiñón, V.P. Herranz, Effects of solution temperature on localized corrosion of high nickel content stainless steels and nickel in chromated LiBr solution. Corros. Sci. 48, 3349–3374 (2006)
G.C. Palit, V. Kain, H.S. Gadiyar, Electrochemical investigations of pitting corrosion in nitrogen-bearing type 316LN stainless steel. Corrosion 49, 977–991 (1993). https://doi.org/10.5006/1.3316025
C.-C. Shih, C.-M. Shih, Y.-Y. Su, L.H.J. Su, M.-S. Chang, S.-J. Lin, Effect of Surface oxide properties on corrosion resistance of 316L stainless steel for biomedical applications. Corros. Sci. 46, 427–441 (2004)
M.G. Fontana, Corrosion Engineering, 3rd edn. (McGraw-Higher Education, London, 1987), p. 0071003606
J.F. Moulder, W.F. Stickle, P.E. Sobol, K.D. Bomben, in Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data, ed. by J. Chastain, R.C. King Jr. (Physical Electronics, Eden Prairie, 1995)
M.C. Biesinger, B.P. Payne, A.P. Grosvenor, L.W.M. Lau, A.R. Gerson, R.S.C. Smart, Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 257, 2717–2730 (2011). https://doi.org/10.1016/j.apsusc.2010.10.051
A. Kapoor, S. Bahadur, Transfer film bonding and wear studies on CuS-nylon composite sliding against steel. Tribol. Int. 27, 323–329 (1994)
M. Raposo, Q. Ferreira, P.A. Ribeiro, A guide for atomic force microscopy analysis of soft-condensed matter. Mod. Res. Educ. Top. Microsc. 1, 758–769 (2007)
K.M. Emran, Effects of concentration and temperature on the corrosion properties of the Fe–Ni–Mn alloy in HCl solutions. Res. Chem. Intermed. 41, 3583–3596 (2015). https://doi.org/10.1007/s11164-013-1473-9
A. Hamdy, N.S. El-Gendy, Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium. Egypt. J. Pet. 22, 17–25 (2013). https://doi.org/10.1016/j.ejpe.2012.06.002
Acknowledgements
The authors would like to thank Dr. Hartmann Thomas from Vacuumschmelze company for providing the specimens. Also, the authors would like to thank Mr. Abdallah Jaber of physical department for conducting surface measurements of the study samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Emran, K.M., Al-Harbi, A.K. Different Impacts on the Corrosion Behavior of Metal–Metal Glassy Alloys in Sulfuric Acid. J Inorg Organomet Polym 29, 144–158 (2019). https://doi.org/10.1007/s10904-018-0974-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-018-0974-9