Abstract
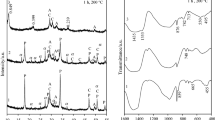
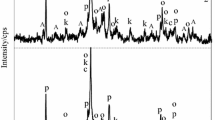
A new form of amorphous silica SiO2 synthesizable by polycondensation of the silicic acids previously recovered from serpentines (Mg(Fe))6[Si4O10](OH)8 was used as a raw material for the β-wollastonite (β-CaSiO3) production. The investigations of this paper are focused on the study of sodium hydroxide NaOH influence on the reaction between this silica and calcium hydroxide Ca(OH)2, and the procedure of calcium silicates production. Experimental studies have shown that in the presence of NaOH the interaction between the compounds in the SiO2–CaO–H2O system can be initiated by stirring under normal conditions at the temperature of 95 °C in air at ambient pressure within 2 h resulting in the formation of such intermediate amorphous calcium hydro- and hydroxosilicates that begin transforming into calcium silicates, especially β-CaSiO3, up to 815 °C on heating. The temperature-induced processes and phase transformations in these intermediates were investigated by using thermal and X-ray diffraction analyses; the synthesized product was studied by infrared spectroscopy and electron microscopy. The data derived from the experimental investigations allow concluding that the existence of weak (unsaturated) Si–O(Si) bonds in the structure of the amorphous silica is the main microscopic factor accounting for the small energy input and allowing to avoid the energy-consuming autoclave treatment generally applicable for the β-wollastonite manufacture. Reacting with the SiO2, NaOH cuts these weak bonds thereby releasing chain-like silicate anions and promoting the formation of the product largely comprised of β-wollastonite with particle dimensions up to 4 μm.










Similar content being viewed by others
References
Q. Ding, Z. Zhang, C. Wang, J. Jiang, G. Li, K. Mai, J. Therm. Anal. Calorim. 115, 675 (2014)
R. Morsy, R. Abuelkhair, T. Elnimr, Silicon 1 (2014). doi:10.1007/s12633-014-9243-x
R.P. Sreekanth Chakradhar, B.M. Nagabhushana, G.T. Chandrappa, K.P. Ramesh, J.L. Rao, Mater. Chem. Phys. 95, 169 (2006)
Y.-H. Yun, C.-H. Yoon, Y.-H. Kim, C.-K. Kim, S.-B. Kim, J.-T. Kwon, et al., Ceram. Int. 28, 503 (2002)
N.S. Negmatov, Z.Z. Abdullaev, Glass Ceram. 58, 396 (2001)
T. Kokubo, Biomaterials 12, 155 (1991)
L. Jingjiang, W. Xiufen, G. Qipeng, J. Appl. Polym. Sci. 41, 2829 (1990)
H. Wu, J. Yang, H.W. Ma, M.W. Wang, Integr. Ferroelectr. 146, 144 (2013)
A. Yazdani, H.R. Rezaie, H. Ghassai, M. Mahmoudian, J. Ceram. Process. Res. 14, 12 (2013)
K. Lin, J. Chang, X. Liu, C. Ning, Int. J. Appl. Ceram. Technol. 7, 178 (2010)
J. Wu, Y.-J. Zhu, G.-F. Cheng, Y.-H. Huang, Mater. Res. Bull. 45, 509 (2010)
A. Yazdani, H.R. Rezaie, H. Ghassai, J. Ceram. Process. Res. 11, 348 (2010)
K.G. Grigoryan, G.A. Arutunyan, L.G. Baginova, G.O. Grigoryan, Theor. Found. Chem. Eng. 42, 583 (2008)
K. Lin, J. Chang, G. Chen, M. Ruan, C. Ning, J. Cryst. Growth 300, 267 (2007)
K. Lin, J. Chang, J. Lu, Mater. Lett. 60, 3007 (2006)
G. Matekonis, R. Šiaučiūnas, D. Vaičiukynienė, Mater. Sci. 16, 242 (2010)
N.O. Zulumyan, A.R. Isaakyan, Z.G. Oganesyan, Russ. J. Appl. Chem. 80, 1020 (2007)
A. Torosyan, N. Zulumyan, Z. Hovhannisyan, S. Ghazaryan (eds.), The Influence of Mechanical Processing on the Process of Thermal Reduction of SiO 2 by Al Powder. EPD Congress 2005 as held at the 2005 TMS Annual Meeting, 2005
N. Zulumyan, A. Isahakyan, Z. Hovhannisyan, A. Torosyan, Magnes. Technol. 351, 2006 (2006)
N.O. Zulumyan, A.R. Isaakyan, P.A. Pirumyan, A.A. Beglaryan, Russ. J. Phys. Chem. A 84, 700 (2010)
A.R. Isahakyan, H.A. Beglaryan, P.A. Pirumyan, L.R. Papakhchyan, N.H. Zulumyan, Russ. J. Phys. Chem. A 85, 72 (2011)
W. Deer, R. Howie, J. Zussman, Rock-Forming Minerals. Sheet Silicates, vol. 3 (Longman, London, 1962)
N. Zulumyan, A. Mirgorodski, A. Isahakyan, H. Beglaryan, J. Therm. Anal. Calorim. 115, 1003 (2014)
N.H. Zulumyan, L.R. Papakhchyan, A.M. Terzyan, H.A. Beglaryan, A.R. Isahakyan, Theor. Found. Chem. Eng. 47, 185 (2013)
N.H. Zulumyan, L.R. Papakhchyan, A.R. Isahakyan, H.A. Beglaryan, A.M. Terzyan, Geochem. Int. 49, 937 (2011)
N.H. Zulumyan, L.R. Papakhchyan, A.R. Isahakyan, H.A. Beglaryan, S.G. Aloyan, Russ. J. Phys. Chem. A 86, 1008 (2012)
N.H. Zulumyan, L.R. Papakhchyan, A.R. Isahakyan, H.A. Beglaryan, S.G. Aloyan, Russ. J. Phys. Chem. A 86, 1887 (2012)
H. Kirschenbaum, The Classical Chemical Analysis of Silicate Rocks: the Old and the New, vol. 1547. U.S.: Geological Survey Bulletin, 1983.
E. Menéndez, L. Vega, C. Andrade, J. Therm. Anal. Calorim. 110, 203 (2012)
J. Zelić, D. Rušić, R. Krstulović, J. Therm. Anal. Calorim. 67, 613 (2002)
J. Tolliday, Nature 182, 1012 (1958)
A.N. Lazarev, Vibrational Spectra and Structure of Silicates (Springer, New York, 1995), p. 302
N.V. Chukanov, Infrared spectra of mineral species (Springer, Dordrecht, 2014), p. 1726
Acknowledgements
This work was supported by the RA MES State Committee of Science, in the frames of the research project no. 16YR-1D025.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zulumyan, N., Isahakyan, A., Beglaryan, H. et al. The Influence of NaOH on the Synthesis of Calcium Silicates. J Inorg Organomet Polym 27, 1323–1332 (2017). https://doi.org/10.1007/s10904-017-0586-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0586-9