Abstract
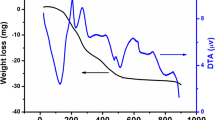
Perovskite LaCr1−xCoxO3 (0 ≤ x ≤ 0.5) oxides synthesized by co precipitation method were investigated. X-ray diffraction, thermo gravimetric and differential thermal analysis, Fourier transform infrared spectroscopy, scanning electron microscopy (SEM) and electrochemical measurements, were used to characterize the structure, morphology, electrochemical properties of the samples. The studied compounds have orthorhombic and rhombohedral systems in the ranges (0 ≤ x ≤ 0.2) and (0.3 ≤ x ≤ 0.5) respectively. Thermal analysis results indicate that the pure phase was obtained at temperature above 800 °C. The structure and morphology of the samples characterized by SEM measurements indicate that particles have nearly spherical shapes and are agglomerated. The electrochemical measurements indicate that the catalytic activity is strongly influenced by cobalt doping. The highest electrode performance is achieved with large cobalt content.











Similar content being viewed by others
References
S. Geller, J. Chem. Phys. 24, 1236 (1956)
S. Geller, J. Acta Crystallogr. 10, 243 (1957)
N. Russo, D. Fino, G. Saracco, V. Specchia, J. Catal. 229, 459 (2005)
M. Penã, J. Fierro, J. Chem. Rev. 101, 1981 (2001)
K. Hilpert, R.W. Steinbrech, F. Borromand, O. Teller, J. Eur. Ceram. Soc. 23, 3009 (2003)
R. Koc, H.U. Anderson, J. Eur. Ceram. Soc. 15, 867 (1995)
S.R. Sehlin, H.U. Anderson, D.M. Sparlin, Solid State Ionics 78, 235 (1995)
J. Sfeir, P.A. Buffat, P. Mockli, N. Xanthopoulos, R. Vasquez, H.J. Mathieu, J. Van herle, K.R. Thampi, J. Catal. 202, 229 (2001)
M. Oishi, K. Yashiro, J. Oh Hong, Y. Nigara, T. Kawada, J. Mizusaki, Solid State Ionics 178, 307 (2007)
H.P.S. Corrêa, C.O. Paiva-Santos, L.F. Setz, L.G. Martinez, S.R.H. Mello-Castanho, M.T.D. Orlando, Powder Diffr. Suppl 23(2), 18 (2008)
A. Bonet, M. Bben, N. Travitzhy, P. Greil, J. Am. Ceram. Soc. 99(3), 917 (2016)
M. Nazari, N. Ghasemi, H. Maddah, M.M. Moltagh, J. Nanostruct. Chem. 4, 99 (2014)
L.E. Crepaldi, C.P. Pavan, B.J. Valim, J. Brraz. Chem. Soc 11(1), 64 (2000)
L.P. Rivas-Vàzqueza, J.C. Rendón-Angeles, J.L. Rodrĭguez-Galicia, K. Zhub, K. Yanagisawa, Solid State Ionics 172, 389 (2004)
P. Duran, J. Tartaj, F. Capel, C. Moure, J. Eur. Ceram. Soc. 24, 2619 (2004)
S. Bilger, G. Blab, R. Forthman, J. Eur. Ceram. Soc. 17, 1027 (1997)
S. Biamino, C. Badini, J. Eur. Ceram. Soc. 24, 3021 (2004)
G. Silva, J. Santos, D. Martinelli, A. Pedrosa, M. de Souza, D. Melo, J. Mater. Sci. Appl. 1, 39 (2010)
S.M. de Lima, J.M. Assaf, J. Mater. Res. 5, 329 (2002)
Z.Q. Tian, W. Huang, Y. Liang, Ceram. Int. 10, 1016 (2008)
S.K. Behera, P.K. Sahu, S.K. Pratihar, S. Bhattacharyya, J. Mater. Lett. 58, 3710 (2004)
S. Bhattacharyya, S.K. Pratihar, R.K. Sinha, R.C. Behera, R.I. Ganguly, J. Mater. Lett. 53, 425 (2002)
P.R.N. Silva, A.B. Soares, Ecl. Quim. Sao Paulo 34, 31 (2009)
K. Rida, A. Benabbas, F. Bouremmad, M.A. Pena, E. Sastre, A. Martinez-Arias, J. Appl. Catal. 327, 173 (2007)
K. Rida, A. Benabbas, F. Bouremmad, M.A. Pena, E. Sastre, A. Martinez, Arias. J. Appl. Catal. 84, 457 (2008)
A. Davydov, Infrared Spectroscopy of Adsorbed Species on the Surface of Transition Metal Oxides (Wiley, England, 1990)
H. Cui, M. Zayat, D. Levy, J. Non-Cryst., Solids 352, 3035 (2006)
D.S. Melo, E.P. Marinho, L.E.B. Soledade, D.M.A. Melo, S.J.G. Lima, E. Longo, I.M.G. Santos, A.G. Souza, J. Mater. Sci. 43, 551 (2008)
A. Baranauskas, D. Jasaitis, A. Kareiva, Vibr. Spectrosc. 28, 263 (2002)
L. Djoudi, M. Omari, J. Inorg. Organomet. Polym. 25, 796 (2015)
J. Livage, M. Henry, C. Sanchez, Prog. J. Solid State Chem. 18, 259 (1988)
C.J. Brinker, G.W. Scherrer, Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing (Academic Press Inc, New York, 1990), p. 59
B. Schrader, Infrared Raman Spectroscopy: Methods and Applications (VCH, Weinheim, 1995)
T. Vaz, A.V. Salker, Mater. Sci. Eng. B 143, 81 (2007)
R.D. Shannon, Acta Crystallogr. Sect. A 32, 751 (1976)
L. Bedel, A.C. Roger, C. Estournes, A. Kiennemann, Catal. Today 85, 207–218 (2003)
N. Escalona, S. Fuentealba, G. Pecchi, Appl. Catal. A 381, 253 (2010)
G. Pecchi, C. Campos, O. Pena, L.E. Cadus, J. Mol. Catal. A 158, 166 (2008)
S.A. Howard, J. Yau, H.U. Anderson, J. Am. Ceram. Soc. 75, 1685 (1991)
I.O. Troyanchuka, D.V. Karpinski Ïa, V.M. Dobryanski Ïa, Y.A. Fedotovab, H. Szymczakc, J. Exp. Theor. Physics. 100(6), 1121 (2005)
N.A. Merino, B. Barbero, P. Ruiz, L.E. Cadús, J. Catal. 240, 245 (2006)
H. Provendier, C. Petit, C. Estournes, S. Libs, A. Kienemann, Appl. Catal. A 180, 163 (1999)
A. Zuev, L. Singheiser, K. Hilpert, Solid State Ionics 147, 1 (2002)
B.D. Cullity, Elements of X-ray Diffractions (Addison Wesley, Reading, 1978)
A. Weidenkaff, S.G. Ebbinghaus, T. Lippert, J. Chem. Mater. 14, 1797 (2002)
Z. Junwu, S. Xiaojie, W. Yanping, W. Xin, Y. Xujie, L. Lude, J. Rare Earths 25, 601 (2007)
G.B. Jung, T.J. Huang, M.H. Huang, C.L. Chang, J. Mater. Sci. 36, 5839 (2001)
M. Diafi, M. Omari, Bol. Soc. Esp. Ceram. Vidr. 51(6), 337 (2012)
S. Trasatti, in Electrochemistry of Novel Materials, ed. by J. Lipkowski, P.N. Ross (VCH, New York, 1994), p. 207
L.G. Tejuca, J.L.G. Fierro, J.M. Tascon, D.D. Eley, H. Pines, P.B. Weisz, Advances in Catalysis (Academic Press, New York, 1989), p. 237
E.J.M. O’Sullivan, E.J. Calvo, in Comprehensive Chemical Kinetics, ed. by R.G. Compton (Elsevier, Amsterdam, 1987), p. 274
H. Wendt, G. Imarisio, J. Appl, Electrochem. 18, 1 (1988)
W. Li, X. Wang, Z. Chen, M. Waje, Y. Yan, J. Phys. Chem. B 110, 15353 (2006)
R.N. Singh, A. Singh, D. Mishra, Anindita, P. Chartier. J. Power Sources 185, 776 (2008)
B. Lal, M.K. Raghunandan, M. Gupta, R.N. Singh, Int. J. Hydrogen Energy 30, 723 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madoui, N., Omari, M. Synthesis and Electrochemical Properties of LaCr1−xCoxO3 (0 ≤ x ≤ 0.5) via Co-precipitation Method. J Inorg Organomet Polym 26, 1005–1013 (2016). https://doi.org/10.1007/s10904-016-0398-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-016-0398-3