Abstract
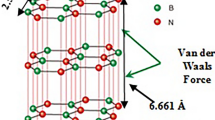
In this paper, by using polymer network different morphologies of hexagonal boron nitride (h-BN) was synthesized. Boric acid (H3BO3) and borax (Na2B4O7·10H2O) mix was used as a source of boron, and urea (CO(NH2)2) was used as a nitrogen source, for the preparation of high crystallinity globular h-BN. The experiment ensured the synthesis mechanism and the optimum synthesis process. The research showed that the synthesis mechanism of h-BN was that borax and boric acid were heated to decomposed into sodium oxide (Na2O) and boron oxide (B2O3). In flowing ammonia (NH3) atmosphere B2O3 transformed into h-BN again; with the increase of the synthesis temperature, h-BN crystallinity increased gradually. The optimum synthesis temperature of high crystallinity spherical h-BN was 800 °C. Graphitization index was 3.87 which was <5 and h-BN was in good crystallization range.






Similar content being viewed by others
References
R.T. Paine, C.K. Narula, Synthetic routes to boron nitride. Chem. Rev. 90, 73–91 (1990)
K. Watanabe, T. Taniguchi, H. Kanda, Direct-bandgap properties and evidence for ultraviolet lasing of hexagonal boron nitride single crystal. Nat. Mater. 3, 404–409 (2004)
V.L. Solozhenko, V.Z. Turkevich, Kinetics of cBN crystallization in the Li3N-BN system at 6.6 GPa. Diam. Relat. Mater. 7, 43–46 (1998)
S.A. Kulinich, A.N. Zhukov, L.G. Sevast’yanova et al., On some alkali-and alkaline-earth-metal boron nitrides, unsaturated with boron. Diam. Relat. Mater. 8, 2152–2158 (1999)
R. Haubner, M. Wilhelm, R. Weissenbacher et al., Boron nitrides-properties, synthesis and applications (Springer, Berlin Heidelberg, 2002)
G.Y. Zhang, C.H. Chen, The development of boron nitride fiber. Polym Mater Sci Eng 14, 94–96 (1998). (In Chinese)
S.G. Guo, B. Lv, J.S. Wang et al., Boron nitride synthesis and application research. Shandong Mech 6, 16–19 (2004). (In Chinese)
H. Kitahara, T. Oku, T. Hirano et al., Synthesis and characterization of cobalt nanoparticles encapsulated in boron nitride nanocages. Diam. Relat. Mater. 10, 1210–1213 (2001)
C.J.H. Jacobsen, Boron nitride: a novel support for ruthenium-based ammonia synthesis catalysts. J. Catal. 200, 1–3 (2001)
J. Eichler, C. Lesniak, Boron nitride (BN) and BN composites for high- temperature applications. J. Eur. Ceram. Soc. 28, 1105–1109 (2008)
R. Ma, Y. Bando, H. Zhu et al., Hydrogen uptake in boron nitride nanotubes at room temperature. J. Am. Chem. Soc. 124, 7672–7673 (2002)
R.S. Pease, An X-ray study of boron nitride. Acta Crystallogr. A 5, 356–361 (1952)
A. Vinu, M. Terrones, D. Golberg et al., Synthesis of mesoporous BN and BCN exhibiting large surface areas via templating methods. Chem. Mater. 17, 5887–5890 (2005)
L. Zhu, M. Tan, G. Lian et al., Hydrothermal hot-pressing induced phase transition in hexagonal boron nitride. Solid State Sci. 11, 1283–1287 (2009)
M. Corso, W. Auwärter, M. Muntwiler et al., Boron nitride nanomesh. Science 303, 217–220 (2004)
S. Kurita, M. Nakashima, H. Takebe et al., Pressureless sintering of h-BN for Continuous temperature measurement of melts. J. Min. Mater. Process. Inst. Jpn. 105, 201–204 (1989)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 51372156), the Liaoning Province Nature Science Foundation (No. L2013455) and the Scientific and Technological Foundation of Shenyang City (No. F13-316-1-46).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, N., Zhang, T., Kan, H. et al. The Research of the Synthesis Mechanism and Synthesis Process of High Crystallinity Globular h-BN. J Inorg Organomet Polym 25, 1495–1501 (2015). https://doi.org/10.1007/s10904-015-0268-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-015-0268-4