Abstract
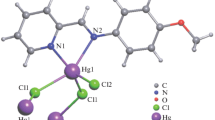
Three new mercury(II) coordination monomers, HgLX3 [X = Cl− (1) and Br− (2)], and polymer, [HgLI2]n, (3), L = 5-methyl-5-(3-pyridyl)-2,4-imidazolidenedione, have been synthesized and characterized by elemental analysis (C, H, and N %) and flame atomic absorption (Hg %), IR, and NMR spectroscopy. Complexes 1 and 2 have the same framework structure while the structure of complex 3 is an infinite 2D layers. According to X-ray diffraction analysis, complex 3 crystallizes in monoclinic system. In this polymeric complex, [Hg(C9H9N3O2)I(μ-I)2]n, HgII atom is coordinated by one L and three I− anions with a distorted tetrahedral geometry; one I− anion is in a monodentate coordination mode and the other two are bridging, linking HgII atoms into a one-dimensional chain. Classical hydrogen-bonding interactions (O···H–N) between adjacent ligands result in a zigzag type arrangement of one chain while consecutive polymeric chains are linked by non-classical ones (O···H–C) to form 2D supramolecular layers. The results indicate that the flexible ligand can form complexes with varied structures. In addition, the species of the halide were found to have great impact on the structure of the complexes. Antibacterial activity of L and the corresponding complexes investigated against six species of microorganisms. Testing was performed by disk diffusion method, and minimum inhibitory concentrations have been determined. Noteworthy antimicrobial activities for these complexes were observed.



Similar content being viewed by others
References
A. Morsali, M.Y. Masoomi, Coord. Chem. Rev. 253, 1882 (2009)
G. Wu, X.-F. Wang, T.-A. Okamura, W.-Y. Sun, N. Ueyama, Inorg. Chem. 45, 8523 (2006)
S.J. Sabounchei, P. Shahriary, S. Salehzadeh, Y. Gholiee, H.R. Khavasi, J. Mol. Struct. 1051, 15 (2013)
B.-C. Tzeng, Y.-C. Huang, B.-S. Chen, W.-M. Wu, S.-Y. Lee, G.-H. Lee, S.-M. Peng, S.-M. Peng, Inorg. Chem. 46, 186 (2007)
A. Castiñeiras, R. Carballo, T. Pérez, Polyhedron 20, 441 (2001)
Y. Niu, Y. Song, H. Hou, Y. Zhu, Inorg. Chim. Acta 355, 151 (2003)
P. Nockemann, G. Meyer, Acta Crystallogr. Sect E E60, m749 (2004)
Y.-Y. Niu, Y.-L. Song, J. Wu, H. Hou, Y. Zhu, X. Wang, Inorg. Chem. Commun. 7, 471 (2004)
Y.-H. Shen, J.-G. Liu, D.-J. Xu, Acta Crystallogr. Sect E E61, m1880 (2005)
A. Jouaiti, N. Kyritsakas, J.-M. Planeix, M.W. Hosseini, CrystEngComm. 8, 883 (2006)
A. Hameau, F. Guyon, M. Knorr, M. Enescu, C. Strohmann, Chem. Mon. 137, 545 (2006)
J. Pansanel, A. Jouaiti, S. Ferlay, M.W. Hosseini, J.-M. Planeix, N. Kyritsakas, N. J. Chem. 30, 71 (2006)
T.J. Burchell, D.J. Eisler, R.J. Puddephatt, J. Mol. Struct. 796, 47 (2006)
G. Mahmoudi, A. Morsali, L.-G. Zhu, Polyhedron 26, 2885 (2007)
X.-P. Li, J.-Y. Zhang, Y. Liu, M. Pan, S.-R. Zheng, B.-S. Kang, C.-Y. Su, Inorg. Chim. Acta 360, 2990 (2007)
Y.-G. Zhu, F. Gao, Acta Crystallogr. Sect E E63, m778 (2007)
M.M. Ebrahim, H. Stoeckli-Evans, K. Panchanatheswaran, Polyhedron 26, 3491 (2007)
G. Mahmoudi, A. Morsali, M. Zeller, Solid State Sci. 10, 283 (2008)
G. Mahmoudi, A. Morsali, Polyhedron 27, 1070 (2008)
G. Mahmoudi, A. Morsali, CrystEngComm 11, 1868 (2009)
A.A. Khandar, V.T. Yilmaz, F. Costantino, S. Gumus, S.A. Hosseini-Yazdi, G. Mahmoudi, Inorg. Chim. Acta 394, 36 (2013)
A.-Q. Wu, Y. Li, F.-K. Zheng, G.-C. Guo, J.-S. Huang, Cryst. Growth Des. 6, 444 (2006)
N.M. Aghatabay, A. Neshat, T. Karabiyik, M. Somer, D. Haciu, B. Dülger, Eur. J. Med. Chem. 42, 205 (2007)
M. Abd El-Hady, R. Zaky, K. Ibrahim, E. Gomaa, J. Mol. Struct. 1016, 169 (2012)
I. Ahmed, M. Kassem, Spectrochim. Acta A 77, 359 (2010)
S.J. Sabounchei, P. Shahriary, Curr. Top. Med. Chem. 13, 3026 (2013)
S.J. Sabounchei, P. Shahriary, Y. Gholiee, S. Salehzadeh, H.R. Khavasi, A. Chehregani, Inorg. Chim. Acta 409, 265 (2014)
S.J. Sabounchei, P. Shahriary, S. Salehzadeh, Y. Gholiee, D. Nematollahi, A. Chehregani, A. Amani, New J. Chem. 38, 1199 (2014)
COSMO, Bruker AXS Inc., Madison, 2005
SAINT, Bruker AXS Inc., Madison, 2005
SADABS, Bruker AXS Inc., Madison, 2005
M.C. Burla, R. Caliandro, M. Camalli, B. Carrozzini, G.L. Cascarano, L. De Caro, C. Giacovazzo, G. Polidori, R. Spagna, J. Appl. Crystallogr. 38, 381 (2005)
G.M. Sheldrick, SHELX97 (University of Göttingen, Germany, 1997)
B.A. Forbes, D.F. Sahm, A.S. Weissfeld, E.A. Trevino, in Methods for testing antimicrobial effectiveness, ed. by E.J. Baron, L.R. Peterson, S.M. Finegold. Bailey and Scott's Diagnostic Microbiology (Mosby Co., St Louis, Missouri, 1990), p. 171
A.D. Russel, J.R. Furr, J. Appl. Bacteriol. 43, 23 (1977)
M. Khan, A. Omoloso, Fitoterapia 74, 695 (2003)
N.C.f.C.L. Standards, Wayne, 2008
C.C. Chu, P. Teague, J. Org. Chem. 23, 1578 (1958)
X.-F. Wang, Y. Lv, T.-A. Okamura, H. Kawaguchi, G. Wu, W.-Y. Sun, N. Ueyama, Cryst. Growth Des. 7, 1125 (2007)
S.J. Sabounchei, F.A. Bagherjeri, C. Boskovic, R.W. Gable, R. Karamian, M. Asadbegy, J. Mol. Struct. 1034, 265 (2013)
S.J. Sabounchei, F.A. Bagherjeri, C. Boskovic, R.W. Gable, R. Karamian, M. Asadbegy, Polyhedron 53, 1 (2013)
N. Dharmaraj, P. Viswanathamurthi, K. Natarajan, Transition Met. Chem. 26, 105 (2001)
M. Tümer, D. Ekinci, F. Tümer, A. Bulut, Spectrochim. Acta A 67, 916 (2007)
Acknowledgments
We are grateful to Bu-Ali Sina University for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10904_2015_206_MOESM1_ESM.doc
CCDC 983968 contains the supplementary crystallographic data for 3. This data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. (DOC 4379 kb)
Rights and permissions
About this article
Cite this article
Sabounchei, S.J., Shahriary, P., Rudbari, H.A. et al. Synthesis and Characterization of Monomeric and Polymeric Hg(II) Complexes with 5-Methyl-5-(3-pyridyl)-2,4-imidazolidenedione Showing a Wide Spectrum of Effective Antibacterial Activities. J Inorg Organomet Polym 25, 1032–1039 (2015). https://doi.org/10.1007/s10904-015-0206-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-015-0206-5