Abstract
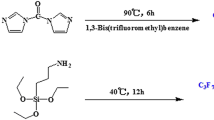
Poly(silsesquioxane)methylsiloxane (PSS) was prepared by the hydrosilylation and hydrolysis reactions using trichlorovinylsilane and dimethyldichlorosilane as starting materials. Poly(fluorosilsesquioxane)methylsiloxane (FPSS) was obtained via a single electron transfer addition reaction of PSS with perfluorobutyl iodide followed by Zn-mediated reduction of iodide. FT-IR, 1H NMR, 19F NMR and 13C NMR indicated the structures of PSS and FPSS. The surface chemical composition and element distribution of polymer films were measured using X-ray Photoelectron Spectroscopy. In addition, Atomic Force Microscopy images showed that there were numerous pinnacles that were generated on the rough surface of films by the segregation of fluoroalkyl side chains at the polymer-air interface. The relative static contact angles of PSS and FPSS films toward water were 105.1° and 119.3°, respectively. The FPSS was characterized to possess of better water repellent property because of the segregation and enrichment of fluoroalkyl chains on the surface of FPSS.
Graphical Abstract









Similar content being viewed by others
References
D.A. Loy, K.J. Shea, Chem. Rev. 95, 1431–1442 (1995)
H.B. Buergi, K.W. Toernroos, G. Calzaferri, H. Buergy, Inorg. Chem. 32, 4914–4919 (1993)
C.X. Zhang, R.M. Laine, J. Organomet. Chem. 521, 199–201 (1996)
A. Tsuchida, F.G. Sernetz, H. Frey, R. Mulhaupt, C. Bolln, Macromolecules. 30, 2818–2824 (1997)
A. Lee, J.D. Lichtenhan, Macromolecues 31, 4970–4974 (1998)
F.J. Feher, D.A. Newman, J.F. Walzer, J. Am. Chem. Soc. 111, 1741–1748 (1989)
N. Maxim, A. Overweg, P.J. Kooyman, H.M. Jos et al., J. Phys. Chem. B. 106, 2203–2209 (2002)
C. Bonhomme, J.P. Toledano, J. Maquet, J. Livage, C.L. Bonhomme, J. Chem. Soc. 9, 1617–1626 (1997)
A.R. Bassindale, M. Pourny, P.G. Taylor, M.B. Hursthouse, M.E. Light, Angew. Chem. Int. Ed. 42, 3488–3490 (2003)
C.X. Zhang, C. Bonhomme, R.M. Laine, C.L. Soles, H.A. Hristov, A.F. Yee, F. Babonneau, J. Am. Chem. Soc. 120, 8380–8391 (1998)
A. Sellinger, R.M. Laine, Macromolecules 29, 2327–2330 (1996)
J. Choi, S.G. Kim, R.M. Laine, Macromolecules 37, 99–109 (2004)
J.W. Choi, R. Tamaki, S.G. Kim, R.M. Laine, Chem. Mater. 15, 3365–3375 (2003)
J.D. Lichtenhan, Comm. Inorg. Chem. 17, 115–130 (1995)
A.J. Barry, W.H. Daudt, J.J. Domicone, J.W. Gilkey, J. Am. Chem. Soc. 77, 4248–4252 (1955)
M.G. Voronkov, V.I. Lavrentyev, Top. Curr. Chem. 102, 199–236 (1982)
K.A. Andrianov, B.A. Lzmaylov, J. Organomet. Chem. 8, 435–437 (1967)
J.J. Schwab, J.D. Lichtenhan, Appl. Organomet. Chem. 12, 707–713 (1998)
H. Kobayashi, Makromol. Chem. 194, 2569–2571 (1993)
S.T. Iacono, A. Vij, W. Grabow, D.W. Smith, Chem. Commun. 47, 4992–4994 (2007)
G.S. Edward, G.B. Alan, F.J. Paul, J.S. Joseph, P.C. Kevin, S.H. Timothy, J.D. Lichtenhan, Appl. Organometal. Chem. 13, 311–327 (1999)
T. Fuchikami, I. Ojima, Tetrahedron Lett. 25, 303–306 (1984)
K. Vonwerner, J. Fluorine Chem. 28, 229–233 (1985)
C.P. Zhang, Q.Y. Chen, Y. Guo, J.C. Xiao, Y.C. Gu, Chem. Soc. Rev. 41, 4536–4559 (2012)
Q.Y. Chen, Z.Y. Yang, J. Fluorine Chem. 28, 399–411 (1985)
Q.Y. Chen, Y.B. He, Z.Y. Yang, J. Fluorine Chem. 34, 255–258 (1986)
Q.Y. Chen, Z.Y. Yang, Acta Chim. Sinica 43, 1118–1120 (1985)
Q.Y. Chen, Z.Y. Yang, Youji Huaxue 1, 41–43 (1986)
Ma, S.M.; Lu, X.Y. J. Chem Soc. Perkin I. 2031-2033 (1990)
X.-C. Zhang, W.-Y. Huang, J. Fluorine Chem. 1, 57–64 (1998)
O.B. Neal, J. Fluorine Chem. 108, 147–175 (2001)
O.B. Neal, J. Fluorine Chem. 93, 1–25 (1999)
K. Yue, C. Liu, K. Guo, X.F. Yu, M.J. Huang, Y.W. Li, W. Chrys, Z.D. Stephen, W.B. Zhang, Macromolecules 45, 8126–8134 (2012)
R.N. Wenzel, Ind. Eng. Chem. 28, 988–994 (1936)
Acknowledgments
Thanks for the financial supports by National Natural Science Foundation of China (51273140), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions are greatly appreciated, and State Key Laboratory for Disaster Prevention and Mitigation Explosion Shock(DPMEIKF 201305).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zang, X., Cai, L., Yuan, Y. et al. Fluoroalkylation of Silsesquioxanes-Modified Polysiloxane and Its Surface Property. J Inorg Organomet Polym 25, 975–981 (2015). https://doi.org/10.1007/s10904-015-0201-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-015-0201-x