Abstract
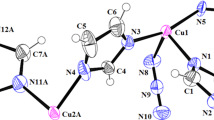
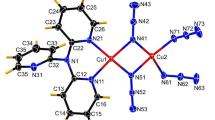
Two one-dimensional copper(II) coordination polymers with different dissymmetrical N,N′-bis(substituted)oxamide ligands were synthesized, namely {[Cu2(dmaepox)(dabt)]pic·H2O}n (1) and {[Cu2(dmapob)(dabt)]NO3·0.6H2O}n (2), where H3dmaepox and H3dmapob stand for N-(2-carboxylatophenyl)-N′-[3-(methylamino)propyl]oxamidate and N-(2-carboxylatophenyl)-N′-[3-(dimethylamino)propyl]oxamidate, respectively, and dabt is 2‚2′-diamino-4‚4′-bithiazole. Polymer 1 was characterized by elemental analyses, IR and electronic spectra and single-crystal X-ray diffraction. The crystal structure reveals that polymer 1 consists of many binuclear copper(II) units bridged by cis-oxamide and carboxylate groups. The two copper(II) ions are located in square-planar and square-pyramidal coordination environments, respectively. The separations of the Cu(II) atoms bridged by oxamide and carboxylate groups are 5.2035(7) and 5.1196(7) Å, respectively. The crystal structure of polymer 2 has been reported in our previous paper. However, its relative properties were not studied. In order to compare the influence of different bridging ligands on the cytotoxicities and DNA-binding properties, the cytotoxicities and reactivities towards DNA of polymers 1 and 2 were investigated. The results suggest that the two polymers can interact with herring sperm DNA in the mode of intercalation with binding affinities following the order of 1 > 2, and this is consistent with their in vitro cytotoxicities.







Similar content being viewed by others
References
Y.B. Zeng, N. Yang, W.S. Liu, N. Tang, J. Inorg. Biochem. 97, 258 (2003)
B. Rosenberg, L. Van Camp, J.E. Trosko, V.H. Mansour, Nature 222, 385 (1969)
E.R. JamiesonandS, J. Lippard, Chem. Rev. 99, 2467 (1999)
E. Wong, C.M. Giandomenico, Chem. Rev. 99, 2451 (1999)
V. Rajendiran, M. Murali, E. Suresh, Dalton Trans. 16, 2157 (2008)
C.Y. Zhou, J. Zhao, Y.B. Wu, C.X. Yin, P. Yang, J. Inorg. Biochem. 101, 10 (2007)
F. Gao, H. Chao, F. Zhou, Y.X. Yuan, B. Peng, L.N. Ji, J. Inorg. Biochem. 100, 1487 (2006)
U. Pindur, M. Haber, K. Sattler, J. Chem. Educ. 70, 263 (1993)
M. Navarro, E.J. Cisneros-Fajardo, A. Sierralta, M. Ferández-Mestre, P. Silva, D. Arrieche, E. Marchán, J. Biol. Inorg. Chem. 8, 401 (2003)
S. Dhar, M. Nethaji, A.R. Chakravarty, Inorg. Chim. Acta 358, 2437 (2005)
A.M. Thomas, M. Nethaji, A.R. Chakravarty, J. Inorg. Boichem. 98, 1087 (2004)
M. Jiang, Y.T. Li, Z.Y. Wu, Z.Q. Liu, C.W. Yan, J. Inorg. Biochem. 103, 833 (2009)
A.M. Thomas, G. Neelakanta, S. Mahadevan, M. Nethaji, A.R. Chakravarty, Eur. J. Inorg. Chem. 2002, 2720 (2002)
E. Dfaz, R. Valenciano, P. Landa, J.L. Arana, J. Gonzalez, Polym. Test 21, 247 (2002)
I. Katime, J.R. Ochoa, J. Appl. Polym. Sci. 34, 1953 (1987)
R. Senthil Kumar, S. Arunachalam, Polyhedron 25, 3113 (2006)
B. Schechter, R. Arnon, M. Wilchek, React. Polym. 25, 167 (1995)
J.J. Nie, Y.T. Li, Z.Y. Wu, X.W. Li, C.W. Yan, Acta Cryst. 66, 327 (2010)
H. Ojima, Y. Yamada, Bull. Chem. Soc. Jpn. 43, 3018 (1970)
G.M. Sheldrick, SHSLXL-97, Program for Crystal Structure Refinement (University of Göttingen, Germany, 1997)
J. Marmur, Mol. J. Biol. 3, 208 (1961)
M.E. Reichmann, S.A. Rice, C.A. Thomas, P.J. Doty, J. Am. Chem. Soc. 76, 3047 (1954)
J.B. Chaires, N. Dattagupta, D.M. Crothers, Biochemistry 21, 3933 (1982)
G. Cohen, H. Eisenberg, Biopolymers 8, 45 (1969)
J.K. Barton, J.M. Goldberg, C.V. Kumar, N.J. Turro, J. Am. Chem. Soc. 108, 2081 (1986)
H. Ojima, K. Nonoyama, Coord. Chem. Rev. 92, 85 (1988)
Y.T. Li, C.W. Yan, H.S. Guan, Polyhedron 22, 3223 (2003)
B. Chand, U. Ray, G. Mostafa, T.H. Lu, C. Sinha, Polyhedron 23, 1669 (2004)
W.J. Geary, Coord. Chem. Rev. 7, 81 (1971)
Y.T. Li, Z.Q. Liu, Z.Y. Wu, J. Inorg. Biochem. 102, 1790 (2008)
R.S. Kumar, S. Arunachalam, Eur. J. Med. Chem. 44, 1878 (2009)
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th edn. (Wiley, New York, 1997)
S.X. Liu, W.S. Liu, M.Y. Tan, K.B. Yu, J. Coord. Chem. 39, 105 (1996)
L.Z. Zhang, W. Gu, B. Li, X. Liu, D.Z. Liao, Inorg. Chem. 46, 622 (2007)
A.B.P. Lever, Inorganic Electronic Spectroscopy (Elsevier Publishing Co, Amsterdam, 1984)
L.M. Duan, F.T. Xie, X.M. Chen, Y. Chen, Y.K. Lu, P. Cheng, J.Q. Xu, Cryst. Growth Des. 6, 1101 (2006)
Y.X. Tong, X.M. Chen, S.W. Ng, Polyhedron 16, 3363 (1997)
A. Wolf, G.H. Shimer Jr, T. Meehan, Biochemistry 26, 6392 (1987)
A.W. Addison, T.N. Rao, J. Reedijk, J. van Rijn, G.C. Verschoor, J. Chem. Soc. Dalton Trans. 1349 (1984)
D. Cremer, J.A. Pople, J. Am. Chem. Soc. 97, 1354 (1975)
C. Jubert, A. Mohamadou, C. Gerard, S. Brandes, A. Tabard, J.P. Barbier, J. Chem. Soc. Dalton Trans. 2660 (2002)
V.A. BloomEeld, D.M. Crothers, I. Tinoco. Physical Chemistry of Nucleic Acids. (Harper and Row, New York, 1974) p. 432
A.M. Pyle, J.P. Rehmann, R. Meshoyrer, C.V. Kumar, N.J. Turro, J.K. Barton, J. Am. Chem. Soc. 111, 3051 (1989)
J.B. LePecq, C. Paoletti, J. Mol. Biol. 27, 87 (1967)
S. Sharma, S.K. Singh, M. Chandra, D.S. Pandey, J. Inorg. Biochem. 99, 458 (2005)
K. Dhara, P. Roy, J. Ratha, M. Manassero, P. Banerjee, Polyhedron 26, 4509 (2007)
S. Anbu, M. Kandaswamy, P. Suthakaran, V. Murugan, B. Varghese, J. Inorg. Biochem. 103, 401 (2009)
O. Stern, M. Volmer, Z. Phys. 20, 183 (1919)
S. Mahadevan, M. Palaniandavar, Inorg. Chem. 37, 693 (1998)
Y.M. Song, P.J. Yang, M.L. Yang, J.W. Kang, S.Q. Qin, B.Q. Lu, Trans. Met. Chem. 28, 712 (2003)
M.T. Carter, M. Rodriguez, A.J. Bard, J. Am. Chem. Soc. 111, 8901 (1989)
S. Satyanarayana, J.C. Dabrowiak, J.B. Chaires, Biochemistry 31, 9319 (1992)
L. Jin, P. Yang, J. Inorg. Biochem. 68, 79 (1997)
Acknowledgments
This project was supposed by the National Natural Science Foundation of China (No.21071133), the Natural Science Foundation of Qingdao City [No. 11-2-4-1-(9)gch)], and the Program for Changjiang Scholars and Innovative Research Team in University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lin-Lin Zhang and Ling-Dong Wang contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zhang, LL., Wang, LD., Li, YT. et al. Synthesis and Structure of 1D Copper(II) Polymers with Dissymmetrical N,N′-Bis(substituted)oxamide Ligands: Cytotoxicity and DNA-Binding Property. J Inorg Organomet Polym 22, 1128–1138 (2012). https://doi.org/10.1007/s10904-012-9705-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-012-9705-9