Abstract
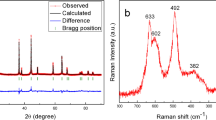
The LiNi0.5Mn1.5O4 cathode material for high power lithium-ion battery is successfully synthesized by sol–gel method. The structure, the morphology and the electrochemical characteristics of the compound are studied by X-ray diffraction (XRD), a field emission scanning electron microscope (FE-SEM), cyclic voltammogram (CV) and charge–discharge techniques, respectively. The results indicate that LiNi0.5Mn1.5O4 sample has cubic spinel structure and its particles crystallized well with submicro size. There are a higher voltage plateau around 4.7 V and a lower voltage plateau above 4 V at 3.3–5.0 V in the charge–discharge curves of LiNi0.5Mn1.5O4, corresponding to two redox peaks of Ni2+/Ni4+ and Mn3+/Mn4+ of its CV respectively. The LiNi0.5Mn1.5O4 with ordered Fd3m space group has 150.57 mAh g−1 initial charge capacity and 139.57 mAh g−1 initial discharge capacity, showing excellent electrochemical performance. A short arc at more high frequency zone from 4.4 V potential emerges in electrochemical impedance spectroscopy (EIS), attributing to oxidative decomposition of the electrolyte at high voltage. The equivalent circuit selected could fit the EIS experiment data very well.







Similar content being viewed by others
References
H.W. Chan, J.G. Duh, S.R. Sheen, J. Power Sources 110, 115 (2003)
P.M. Laura, P.R. Luz, M.R. Rose et al., Electrochim. Acta 3193, 51 (2006)
Y. Hu, Y.H. Liu, Mater. Chem. Phys. 255, 90 (2005)
J. Gong, J.H. Yang, C.H. Wang, Chem. J. Chin. Univ. 2322, 23 (2002)
G.T.K. Fey, W. Li, J.R. Dahn, J. Electrochem. Soc. 2279, 141 (1994)
K.J. Hong, Y.K. Sun, J. Power Sources 427, 109 (2002)
S.H. Park, Y.K. Sun, Electrochim. Acta 431, 50 (2004)
Y. Idemoto, H. Sekine, K. Ui et al., Solid State Ion. 299, 176 (2005)
K.H. Dai, J.Z. Mao, C. Yu, Acta Phys. Chim. Sin. 2130, 26 (2010)
P.P. Pang, Z.Y. Tang, X.L. Yuan, Chin. Battery Ind. 160, 15 (2010)
J.H. Kim, S.T. Myung, Y.K. Sun, Electrochim. Acta 219, 49 (2004)
K. Amine, H. Tukamoto, H. Yasuda et al., J. Electrochem. Soc. 1607, 143 (1996)
L. Guohua, H. Ikuta, T. Uchida et al., J. Electrochem. Soc. 178, 143 (1996)
R. Alcántara, M. Jaraba, P. Lavela et al., Electrochim. Acta 1829, 47 (2002)
Y. Idemoto, I. Narai, N. Koura, J. Power Sources 125, 119–121 (2003)
L.J. Fu, H. Liu, C. Li et al., Prog. Mater. Sci. 881, 50 (2005)
Y.S. Lee, Y.K. Sun, S. Ota et al., Electrochem. Commun. 989, 4 (2002)
L. Yu, Y. Cao, H. Yang et al., J. Solid State Electrochem. 283, 10 (2006)
Y.K. Sun, K.J. Hong, J. Prakash et al., Electrochem. Commun. 344, 4 (2002)
Z.Q. Liu, W.L. Wang, X.M. Liu et al., J. Solid State Chem. 1585, 177 (2004)
H.J. Guo, X.H. Li, Z.X. Wang et al., J. Power Sources 95, 189 (2009)
M.W. Raja, S. Mahanty, R.N. Basu, J. Power Sources 618, 192 (2009)
Y.P. Fu, Y.H. Su, C.H. Lin, Solid State Ion. 137, 166 (2004)
C. Sigala, A.L. La Salle, Y. Piffard et al., J. Electrochem. Soc. A819, 148 (2001)
N. Kamarulazman, R. Yusoff, N. Kamarudin et al., J. Power Sources 274, 188 (2009)
P.S. Bin, S.H. Chul, L. Wan-Gyu et al., J. Power Sources 597, 5 (2008)
Z.Y. Tand, R. Huang, L. Wang, Chem. Ind. Eng. Prog. 31, 25 (2006)
Q.M. Zhong, A. Bonakdarpour, M.J. Zhang et al., J. Electrochem. Soc. 205, 144 (1997)
H.J. Bang, V.S. Donepudi, J. Prakash, Electrochim. Acta 443, 48 (2002)
K. Takahashi, M. Saitoh, M. Sano et al., J. Electrochem. Soc. A173, 151 (2004)
Y. Gao, K. Myrtle, M. Zhang, J.N. Reimers, J.R. Dahn, Phys. Rev. B. 54, 16670 (1996)
T. Zheng, J.R. Dahn, Phys. Bev. B. 3800, 56 (1997)
Y. Gao, K. Myrtle, M. Zhang et al., Phys. Rev. B. 16670, 54 (1996)
J. Molenda, J. Marzec, K. Swierczek et al., Solid State Ion. 297, 1–4 (2004)
M. Kunduraci, G.G. Amatucci, J. Power Sources 359, 1659 (2007)
J.S. Gnanaraj, Y.S. Cohen, M.D. Levi et al., J. Electroanal. Chem. 89, 516 (2001)
D. Aurbach, B. Markovsky, M.D. Levi et al., J. Power Sources 95, 81–82 (1999)
S. Zhang, P. Shi, Electrochim. Acta 1475, 49 (2004)
Acknowledgments
This work was financially supported by the National Key Basic Research Program of China (973) (2009CB220102), Fundamental Research Funds for the Central Universities (2010LKHX03, 2010QNB04, 2010QNB05) and Science and Technology “Climbing” Program of China University of Mining and Technology (ON090237).
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the 1st International Congress on Advanced Materials held in Jinan, PRC, from May 12–17, 2011.
Rights and permissions
About this article
Cite this article
Cui, YL., Sun, Z. & Zhuang, QC. Electrochemical Properties of a 4.7 V-Class LiNi0.5Mn1.5O4 Positive Electrode Material for High Power Li-Ion Battery. J Inorg Organomet Polym 21, 893–899 (2011). https://doi.org/10.1007/s10904-011-9523-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-011-9523-5