Abstract
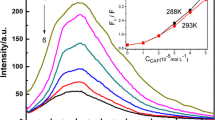
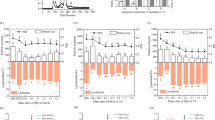
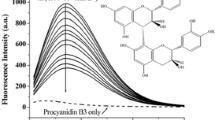
Sinapic acid (SA) and ferulic acid (FA) are bioactive compounds used in the food, pharmaceutical, and cosmetic industries due to their antioxidant properties. In this work, we studied the photophysical properties of SA and FA in different solvents and concentrations and their interactions with caffeine (CF), using ultraviolet–visible (UV–Vis), fluorescence spectroscopy and Fourier transform infrared (FTIR) spectroscopy. The findings show that the quantum yield, fluorescence lifetime, radiative decay rates, and non-radiative decay rates of SA and FA are influenced by the concentrations and solvent polarity. The interaction between SA and FA with CF was also studied using UV–Vis and fluorescence spectroscopy. The results indicate that the CF quenched the fluorescence intensity of SA and FA by static quenching due to the formation of a non-fluorescent complex. The van't Hoff equation suggests that the van der Waals forces and hydrogen bonds force were responsible for the interaction between SA and CF, as indicated by a negative change in enthalpy (\({\Delta {\text{H}}}^{{\text{o}}}\) < 0) and a negative change in entropy (\({\Delta {\text{S}}}^{{\text{o}}}\) < 0). On the other hand, the interaction between FA and CF was primarily controlled by electrostatic force, as indicated by a negative change in enthalpy (\({\Delta {\text{H}}}^{{\text{o}}}\) < 0) and a positive change in entropy (\({\Delta {\text{S}}}^{{\text{o}}}\) > 0). The negative change in Gibbs free energy (\({\Delta {\text{G}}}^{{\text{o}}}\)) indicates that both compounds underwent a spontaneous binding process.















Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
da Silva APG, Sganzerla WG, John OD, Marchiosi R (2023) A comprehensive review of the classification, sources, biosynthesis, and biological properties of hydroxybenzoic and hydroxycinnamic acids. Phytochem Rev. https://doi.org/10.1007/s11101-023-09891-y
Yang J, Chen J, Hao Y, Liu Y (2021) Identification of the DPPH radical scavenging reaction adducts of ferulic acid and sinapic acid and their structure-antioxidant activity relationship. Lwt 146(April):111411. https://doi.org/10.1016/j.lwt.2021.111411
El-Seedi HR et al (2012) Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J Agric Food Chem 60(44):10877–10895. https://doi.org/10.1021/jf301807g
Chen J, Yang J, Ma L, Li J, Shahzad N, Kim CK (2020) Author Correction: Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids (Scientific Reports, (2020), 10, 1, (2611), https://doi.org/10.1038/s41598-020-59451-z). Sci Rep 10(1):1–9. https://doi.org/10.1038/s41598-020-62493-y
Razzaghi-Asl N, Garrido J, Khazraei H, Borges F, Firuzi O (2013) Antioxidant properties of hydroxycinnamic acids: a review of structure- activity relationships. Curr Med Chem 20(36):4436–4450. https://doi.org/10.2174/09298673113209990141
Eroğlu C, Avcı E, Vural H, Kurar E (2018) Anticancer mechanism of Sinapic acid in PC-3 and LNCaP human prostate cancer cell lines. Gene 671(2017):127–134. https://doi.org/10.1016/j.gene.2018.05.049
Lee JY (2018) Anti-inflammatory effects of sinapic acid on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice. Arch Pharm Res 41(2):243–250. https://doi.org/10.1007/s12272-018-1006-6
Chen C (2016) Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid Med Cell Longev. https://doi.org/10.1155/2016/3571614
Borges A, Ferreira C, Saavedra MJ, Simões M (2013) Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist 19(4):256–265. https://doi.org/10.1089/mdr.2012.0244
Asma U, Bertotti ML, Zamai S, Arnold M, Amorati R, Scampicchio M (2024) A kinetic approach to oxygen radical absorbance capacity (ORAC): Restoring order to the antioxidant activity of hydroxycinnamic acids and fruit juices. Antioxidants 13(2):222. https://doi.org/10.3390/antiox13020222
Sharma M et al (2021) Effect of solvent on the photophysical properties of isoxazole derivative of curcumin: a combined spectroscopic and theoretical stud. J Photochem Photobiol A Chem 410(January):113164. https://doi.org/10.1016/j.jphotochem.2021.113164
Hrdlovic P, Donovalova J, Stankovicova H, Gaplovsky A (2010) Influence of polarity of solvents on the spectral properties of bichromophoric coumarins. Molecules 15(12):8915–8932. https://doi.org/10.3390/molecules15128915
Zhang B et al (2017) Highly fluorescent molecularly insulated perylene diimides: Effect of concentration on photophysical properties. Chem Mater 29(19):8395–8403. https://doi.org/10.1021/acs.chemmater.7b02968
Ruiz CC, Hierrezuelo JM, Molina-Bolivar JA (2015) Analysis of the photophysical behavior and rotational-relaxation dynamics of coumarin 6 in nonionic micellar environments: The effect of temperature. Molecules 20(10):19343–19360. https://doi.org/10.3390/molecules201019343
Muthu Prabhu AA, Venkatesh G, Rajendiran N (2010) Spectral characteristics of sulfa drugs: Effect of solvents, pH and β-cyclodextrin. J Solut Chem 39(7):1061–1086. https://doi.org/10.1007/s10953-010-9559-0
ul Huda Z, Mansha A, Asim S, Shahzad A (2022) Effect of pH on fluorescence spectra of coumarin derivatives. J Fluoresc 32(1):57–66. https://doi.org/10.1007/s10895-021-02829-7
Fisone G, Borgkvist A, Usiello A (2004) Caffeine as a psychomotor stimulant: Mechanism of action. Cell Mol Life Sci 61(7–8):857–872. https://doi.org/10.1007/s00018-003-3269-3
Abalo R (2021) Coffee and caffeine consumption for human health. Nutrients 13(9):2918. https://doi.org/10.3390/nu13092918
Motora KG, Beyene TT (2017) Determination of caffeine in raw and roasted coffee beans of Ilu. Indo Am J Pharm Res 7(09):463–470
Mullaicharam A, El-Khider M, Amaresh N (2011) Chemistry and pharmacology of caffeine in different types of tea leaves. Int J Nutr Pharmacol Neurol Dis 1(2):110. https://doi.org/10.4103/2231-0738.84198
Fiani B, Zhu L, Musch BL, Briceno S, Andel R, Sadeq N, Ansari AZ (2021) The neurophysiology of caffeine as a central nervous system stimulant and the resultant effects on cognitive function. Cureus 13(5). https://doi.org/10.7759/cureus.15032
De Pauw K, Roelands B, Van Cutsem J, Marusic U, Torbeyns T, Meeusen R (2017) Electro-physiological changes in the brain induced by caffeine or glucose nasal spray. Psychopharmacology 234(1):53–62. https://doi.org/10.1007/s00213-016-4435-2
Alshahrani SH et al (2023) Dietary caffeine intake is associated with favorable metabolic profile among apparently healthy overweight and obese individuals. BMC Endocr Disord 23(1):1–10. https://doi.org/10.1186/s12902-023-01477-1
Heckman MA, Weil J, de Mejia EG (2010) Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci 75(3):77–87. https://doi.org/10.1111/j.1750-3841.2010.01561.x
Tan A, Bozkurt E, Kara Y (2017) Investigation of solvent effects on photophysical properties of new aminophthalimide derivatives-based on methanesulfonate. J Fluoresc 27(3):981–992. https://doi.org/10.1007/s10895-017-2033-2
Rezazadeh M, Ghiasi R, Jamehbozorgi S (2018) Solvent effects on the structure and spectroscopic properties of the second-generation anticancer drug carboplatin: a theoretical insight. J Struct Chem 59(1):245–251. https://doi.org/10.1134/S0022476618010390
Sherefedin U, Belay A, Kebede A, Asemare S, Woldegiorges K, Kumela AG, Gudishe K (2023) Determination of the ground and excited state dipole moments of ferulic and sinapic acids by solvatochromic effects and density function theory method. AIP Adv 13(10). https://doi.org/10.1063/5.0160608
Asemare S, Belay A, Kebede A, Sherfedin U (2023) Ground and excited state dipole moments of metformin hydrochloride using solvatochromic effects and density functional theory. J Fluoresc. https://doi.org/10.1007/s10895-023-03355-4
Taherpour AA, Mozafai A, Ranjbar S, Taban S (2015) A study of the effects of solvent on structural and conformational properties of ranitidine tautomer forms by DFT method. Struct Chem 26(2):517–529. https://doi.org/10.1007/s11224-014-0510-7
Blaisi J, Averbeck D, Moron J, Bisagni E, Vignv P (1987) Effect of molecular structure on the photophysical properties, the photoreactivity with dna and the photobiological activity of monofunctional pyridopsoralens. Photochem Photobiol 45(4):465–472. https://doi.org/10.1111/j.1751-1097.1987.tb05404.x
Maren TH (1976) Relatons between structure and biological activity of sulfonamides. Annu Rev Pharmacol Toxicol 16(1):309–327. https://doi.org/10.1146/annurev.pa.16.040176.001521
Woldegiorges K, Belay A, Kebede A (2022) Photophysical properties of levofloxacin and norfloxacin drugs and their fluorescence quenching mechanism with caffeine. Spectrosc Lett 55(8):500–513. https://doi.org/10.1080/00387010.2022.2110596
Chakraborty P, Roy B, Bairi P, Nandi AK (2012) Improved mechanical and photophysical properties of chitosan incorporated folic acid gel possessing the characteristics of dye and metal ion absorption. J Mater Chem 22(38):20291–20298. https://doi.org/10.1039/c2jm33995a
Mohi, A. T., & Abd Alreda, H. A. A. (2022). Solvent Effect on the spectral and photophysical properties of the Acridone. In Journal of Physics: Conference Series 2322(1) 012075). https://doi.org/10.1088/1742-6596/2322/1/012075
Trzcionka J, Noirot A, Fabre PL, Chouini-Lalanne N (2005) Comparative study of the photophysical properties of indoprofen photoproducts in relation with their DNA photosensitizing properties. Photochem Photobiol Sci 4(3):298–303. https://doi.org/10.1039/b414654a
Skarga VV, Matrosov AA, Nichugovskiy AI, Negrebetsky VV, Maslov MA, Boldyrev IA, Malakhov MV (2021) pH-Dependent photoinduced interconversion of furocoumaric and furocoumarinic acids. Molecules 26(9):2800. https://doi.org/10.3390/molecules26092800
Berezin MY, Kao J, Achilefu S (2009) PH-dependent optical properties of synthetic fluorescent imidazoles. Chem - A Eur J 15(14):3560–3566. https://doi.org/10.1002/chem.200801784
Ghigo G, Vione D, Berto S (2020) Experimental and theoretical study of the fluorescence emission of ferulic acid: Possible insights into the fluorescence properties of humic substances. Spectrochim Acta - Part A Mol Biomol Spectrosc 228:117587. https://doi.org/10.1016/j.saa.2019.117587
Smyk B, Mȩdza G, Kasparek A, Pyrka M, Gryczynski I, Maciejczyk M (2017) Spectroscopic properties and conformational analysis of methyl ester of sinapic acid in various environments. J Phys Chem B 121(30):7299–7310. https://doi.org/10.1021/acs.jpcb.7b05508
Zhang HM, Tang BP, Wang YQ (2010) The interaction of lysozyme with caffeine, theophylline and theobromine in solution. Mol Biol Rep 37(7):3127–3136. https://doi.org/10.1007/s11033-009-9891-x
Bjørkedal E, Flaten MA (2011) Interaction between expectancies and drug effects: An experimental investigation of placebo analgesia with caffeine as an active placebo. Psychopharmacology 215(3):537–548. https://doi.org/10.1007/s00213-011-2233-4
Miziak B, Błaszczyk B, Chrościńska-Krawczyk M, Czuczwar SJ (2023) Caffeine and Its Interactions with Antiseizure Medications—Is There a Correlation between Preclinical and Clinical Data? Int J Mole Sci 24(24):17569. https://doi.org/10.3390/ijms242417569
Schefer S, Oest M, Rohn S (2021) Interactions between phenolic acids, proteins, and carbohydrates—influence on dough and bread properties. Foods 10(11):1–29. https://doi.org/10.3390/foods10112798
Carrillo JA, Benitez J (2000) Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin Pharmacokinet 39(2):127–153. https://doi.org/10.2165/00003088-200039020-00004
Belay A, Kim HK, Hwang YH (2016) Binding of caffeine with caffeic acid and chlorogenic acid using fluorescence quenching, UV/vis and FTIR spectroscopic techniques. Luminescence 31(2):565–572. https://doi.org/10.1002/bio.2996
Wu Q, Jiang F, Li C, Hu Y, Liu Y (2011) Interaction of caffeine with bovine serum albumin: Determination of binding constants and the. Chin J Chem 29:433–440
Chen L, Zhu M, Hu X, Pan J, Zhang G (2022) Exploring the binding mechanism of ferulic acid and ovalbumin: Insights from spectroscopy, molecular docking and dynamics simulation. J Sci Food Agric 102(9):3835–3846. https://doi.org/10.1002/jsfa.11733
Zhu S, Bai X, Zhu J, Li W, Wang B (2021) Multi-spectral techniques and molecular docking to investigation of the interaction between ferulic acid and pepsin. Spectrochim Acta - Part A Mol Biomol Spectrosc 251:119442. https://doi.org/10.1016/j.saa.2021.119442
Zhang S, Sun X, Qu F, Kong R (2013) Molecular spectroscopic studies on the interaction of ferulic acid with calf thymus DNA. Spectrochim Acta - Part A Mol Biomol Spectrosc 112:78–83. https://doi.org/10.1016/j.saa.2013.04.006
Kang J, Liu Y, Xie MX, Li S, Jiang M, Wang YD (2004) Interactions of human serum albumin with chlorogenic acid and ferulic acid. Biochim Biophys Acta - Gen Subj 1674(2):205–214. https://doi.org/10.1016/j.bbagen.2004.06.021
Zargar S, Alamery S, Bakheit AH, Wani TA (2020) Poziotinib and bovine serum albumin binding characterization and influence of quercetin, rutin, naringenin and sinapic acid on their binding interaction. Spectrochim Acta - Part A Mol Biomol Spectrosc 235:118335. https://doi.org/10.1016/j.saa.2020.118335
Smyk B (2003) Fluorescence study of sinapic acid interaction with bovine serum albumin and egg albumin. J Fluoresc 13(4):349–356. https://doi.org/10.1023/A:1025334029406
Santin LRR et al (2015) Study between solvatochromism and steady-state and time-resolved fluorescence measurements of the Methylene blue in binary mixtures. Dye Pigment 119:12–21. https://doi.org/10.1016/j.dyepig.2015.03.004
Buterbaugh JS et al (1997) Fluorescence lifetime measurements and spectral analysis of adamantyldiazirine. J Am Chem Soc 119(15):3580–3591. https://doi.org/10.1021/ja964022q
Strickler SJ, Berg RA (1962) Relationship between absorption intensity and fluorescence lifetime of molecules. J Chem Phys 37(4):814–822. https://doi.org/10.1063/1.1733166
Bolton JR, Archer MD (1991) Calculation of natural radiative lifetimes from the absorption and fluorescence properties of semiconductors and molecules. J Phys Chem 95(22):8453–8461. https://doi.org/10.1021/j100175a012
Belay A (2010) Measurement of integrated absorption cross-section, oscillator strength and number density of caffeine in coffee beans by integrated absorption coefficient technique. Food Chem 121(2):585–590. https://doi.org/10.1016/j.foodchem.2009.12.052
Georgakopoulou S, Van Grondelle R, Van Der Zwan G (2004) Circular dichroism of carotenoids in bacterial light-harvesting complexes: Experiments and modeling. Biophys J 87(5):3010–3022. https://doi.org/10.1529/biophysj.104.047498
Hilborn RC (1983) Erratum: “Einstein coefficients, cross sections, f values, dipole moments, and all that” [Am. J. Phys. 5 0, 982 (1982)]. Am J Phys 51(5):471–471. https://doi.org/10.1119/1.13515
Abraha A, Gholap AV, Belay A (2016) Study Self-association, Optical Transition Properties and Thermodynamic Properties of Neomycin Sulfate Using UV-Visible Spectroscopy. Int J Biophys 6(2):16–20. https://doi.org/10.5923/j.biophysics.20160602.02
Lakowicz, J. R. (Ed.). (2006). Principles of fluorescence spectroscopy. Boston, MA: springer US. https://doi.org/10.1007/978-0-387-46312-4
Zhang YF, Zhou KL, Lou YY, Pan DQ, Shi JH (2017) Investigation of the binding interaction between estazolam and bovine serum albumin: Multi-spectroscopic methods and molecular docking technique. J Biomol Struct Dyn 35(16):3605–3614. https://doi.org/10.1080/07391102.2016.1264889
Kou SB, Lin ZY, Wang BL, Shi JH, Liu YX (2021) Evaluation of the binding behavior of olmutinib (HM61713) with model transport protein: Insights from spectroscopic and molecular docking studies. J Mol Struct 1224:129024. https://doi.org/10.1016/j.molstruc.2020.129024
Rout J, Swain BC, Mishra PP, Tripathy U (2020) Spectroscopic insight into the interaction of dopamine with spherical gold nanoparticles. J Photochem Photobiol B Biol 203:111770. https://doi.org/10.1016/j.jphotobiol.2019.111770
Panja SK (2021) Dipolar state assisted aggregation induced optical behavior of push-pull Salen-type Schiff base (BIHyDE) in solution. J Mol Struct 1234:130140. https://doi.org/10.1016/j.molstruc.2021.130140
Johnson GE (1980) Effect of concentration on the fluorescence spectra and lifetimes of pyrene in polystyrene films. Macromolecules 13(4):839–844. https://doi.org/10.1021/ma60076a013
Ashoka S, Seetharamappa J, Kandagal PB, Shaikh SMT (2006) Investigation of the interaction between trazodone hydrochloride and bovine serum albumin. J Lumin 121(1):179–186. https://doi.org/10.1016/j.jlumin.2005.12.001
Ross PD, Subramanian S (1981) Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20(11):3096–3102. https://doi.org/10.1021/bi00514a017
Acknowledgements
The authors greatly acknowledge Gurumurthi Thilanaya (Prof) for his contribution to the purchase of Ferulic acid and Sinapic acid samples from Sigma Aldrich (India) for this work.
Funding
This research was not supported by any specific funding.
Author information
Authors and Affiliations
Contributions
Umer Sherefedin: Conceptualization, analysis, writing; editing, writing original draft. Abebe Belay: Conceptualization, supervision, reviewing and editing. Kusse Gudishe: Conceptualization, supervision, reviewing and editing, Alemu Kebede: Supervision, reviewing and editing, Alemayehu Getahun Kumela: Reviewing and editing. Semahegn Asemare: Reviewing and editing.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable as the study does not include any use of animals and humans.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sherefedin, U., Belay, A., Gudishe, K. et al. Photophysical Properties of Sinapic Acid and Ferulic Acid and Their Binding Mechanism with Caffeine. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03689-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03689-7