Abstract
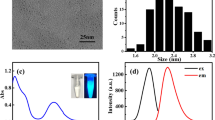
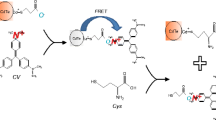
This research introduces a novel fluorescence sensor ‘on-off-on’ employing nitrogen-doped carbon dots (N-CDs) with an ‘on-off-on’ mechanism for the selective and sensitive detection of Hg(II) and L-cysteine (L-Cys). N-CDs was synthesized using citric acid as the carbon precursor and urea as the nitrogen source in dimethylformamide (DMF) solvent, resulting in red emissive characteristics under UV light. Comprehensive spectroscopic analyses, including UV-Vis, fluorescence, FT-IR, XRD, XPS, Raman, and Zeta potential techniques, validated the structural and optical characteristics of the synthesized N-CDs. The maximum excitation and emission of N-CDs were observed at 548 and 622 nm, respectively. The quantum yield of N-CDs was calculated to be 16.1%. The fluorescence of N-CDs effectively quenches upon the addition of Hg(II) due to the strong coordination between Hg(II) and the surface functionalities of N-CDs. Conversely, upon the subsequent addition of L-Cys, the fluorescence of N-CDs was restored. This restoration can be attributed to the stronger affinity of the -SH group in L-Cys towards Hg(II) relative to the surface functionalities of N-CDs. This dual-mode response enabled the detection of Hg(II) and L-Cys with impressive detection limits of 15.1 nM and 8.0 nM, respectively. This sensor methodology effectively detects Hg(II) in lake water samples and L-Cys levels in human urine, with a recovery range between 99 and 101%. Furthermore, the N-CDs demonstrated excellent stability, high sensitivity, and selectivity, making them a promising fluorescence on-off-on probe for both environmental monitoring of Hg(II) and clinical diagnostics of L-Cys.











Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Malik LA, Bashir A, Quraeshi A et al (2019) Detection and removal of heavy metal ions: a review. Environ Chem Lett 17:1495–1521. https://doi.org/10.1007/s10311-019-00891-z
Dong B, Liu X, Dai L et al (2013) Changes of heavy metal speciation during high-solid anaerobic digestion of sewage sludge. Bioresour Technol 131:152–158. https://doi.org/10.1016/j.biortech.2012.12.112
Dubey S, Shri M, Gupta A, Rani V, Chakrabarty D (2018) Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ Chem Lett 16:1169–1192. https://doi.org/10.1007/s10311-018-0741-8
Rehman K, Fatima F, Waheed I et al (2018) Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 119(1):157–184. https://doi.org/10.1002/jcb.26234
Rice KM, Walker EM, Wu M et al (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47(2):74–83. https://doi.org/10.3961/jpmph.2014.47.2.74
Miserendino AR, Guimarães JRD, Schudel G et al (2018) Mercury pollution in Amapá, Brazil: Mercury amalgamation in artisanal and small-scale gold mining or land-cover and land-use changes? ACS Earth Space Chem 2(5):441–450. https://doi.org/10.1021/acsearthspacechem.7b00089
Hilson G (2006) Abatement of mercury pollution in the small-scale gold mining industry: Restructuring the policy and research agendas. Sci Total Environ 362(1–3):1–14. https://doi.org/10.1016/j.scitotenv.2005.09.065
World Health Organization (2008) Guidelines for drinking-water quality, 3rd edn., Geneva
Milic SZ, Potkonjak NI, Gorjanovic SZ et al (2011) A polarographic study of chlorogenic acid and its interaction with some heavy metal ions. Electroanalysis 23:2935–2940. https://doi.org/10.1002/elan.201100476
Xue X, Wang F, Liu X (2008) One-step, room temperature, colorimetric detection of mercury (Hg2+) using DNA/nanoparticle conjugates. J Am Chem Soc 130(11):3244–3245. https://doi.org/10.1021/ja076716c
Ciedziel JV, Gerstenberger S (2004) Determination of total mercury in human hair and animal fur by combustion atomic absorption spectrometry. Talanta 64:918–921. https://doi.org/10.1016/j.talanta.2004.04.013
Anandhakumar S, Mathiyarasu J, Phani KLN (2012) Anodic stripping voltammetric detection of mercury (II) using Au-PEDOT modified carbon paste electrode. Anal Methods 4:2486–2489. https://doi.org/10.1039/c2ay25170a
Wang GL, Zhu XY, Jiao HJ et al (2011) Ultrasensitive and dual functional colorimetric sensors for mercury (II) ions and hydrogen peroxide based on catalytic reduction property of silver nanoparticles. Biosens Bioelectron 3:337–342. https://doi.org/10.1016/j.bios.2011.10.041
Page LE, Zhang X, Jawaid AM et al (2011) Detection of toxic mercury ions using a ratiometric CdSe/ZnS nanocrystal sensor. Chem Commun 47:7773–7775. https://doi.org/10.1039/c1cc11442e
Ball RO, Courtney MG, Pencharz PB (2006) The in vivo sparing of methionine by cysteine in sulfur amino acid requirements in animal models and adult humans. J Nutr 136:1682S–1693S. https://doi.org/10.1093/jn/136.6.1682S
Weerapana E, Wang C, Simon GM et al (2010) Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468:790–797. https://doi.org/10.1038/nature09472
Bulaj G, Kortemme T, Goldenberg DP (1998) Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry 37:8965–8972. https://doi.org/10.1021/bi973101r
Bonifácio VDB, Pereira SA, Serpa J, Vicente JB (2021) Cysteine metabolic circuitries: druggable targets in cancer. Br J Cancer 124:862–879. https://doi.org/10.1038/s41416-020-01156-1
Quijano C, Trujillo M, Castro L, Trostchansky A (2016) Interplay between oxidant species and energy metabolism. Redox Biol 8:28–42. https://doi.org/10.1016/j.redox.2015.11.010
Baba SP, Bhatnagar A (2018) Role of thiols in oxidative stress. Curr Opin Toxicol 7:133–139. https://doi.org/10.1016/j.cotox.2018.03.005
Nidya M, Umadevi M, Rajkumar BJM (2014) Structural, morphological and optical studies of L-cysteine modified silver nanoparticles and its application as a probe for the selective colorimetric detection of Hg2+. Spectrochim Acta - Part A Mol Biomol Spectrosc 133:265–271. https://doi.org/10.1016/j.saa.2014.04.193
Go YM, Jones DP (2011) Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med 50:495–509. https://doi.org/10.1016/j.freeradbiomed.2010.11.029
Lai X, Wang M, Zhu Y et al (2021) ZnO NPs delay the recovery of psoriasis-like skin lesions through promoting nuclear translocation of p-NFκB p65 and cysteine deficiency in keratinocytes. J Hazard Mater 410:124566. https://doi.org/10.1016/j.jhazmat.2020.124566
Puka-Sundvall M, Eriksson P, Nilsson M et al (1995) Neurotoxicity of cysteine: interaction with glutamate. Brain Res 705(95):65–70. https://doi.org/10.1016/0006-8993
Kuśmierek K, Głowacki R, Bald E (2006) Analysis of urine for cysteine, cysteinylglycine, and homocysteine by high-performance liquid chromatography. Anal Bioanal Chem 385:855–860. https://doi.org/10.1007/s00216-006-0454-x
Bald E, Glowacki R, Drzewoski J (2001) Determination by liquid chromatography of free and total cysteine in human urine in the form of its S-quinolinium derivative. J Chromatogr A 913:319–329. https://doi.org/10.1016/S0021-9673(00)01202-4
Ivanov AV, Virus ED, Luzyanin BP et al (2015) Capillary electrophoresis coupled with 1,1’-thiocarbonyldiimidazole derivatizations for the rapid detection of total homocysteine and cysteine in human plasma. J Chromatogr B Anal Technol Biomed Life Sci 1004:30–36. https://doi.org/10.1016/j.jchromb.2015.09.036
Liu T, Li N, Dong JX et al (2016) Fluorescence detection of mercury ions and cysteine based on magnesium and nitrogen co-doped carbon quantum dots and IMPLICATION logic gate operation. Sens Actuators B Chem 231:147–153. https://doi.org/10.1016/j.snb.2016.02
Tajik S, Dourandish Z, Jahani PM et al (2021) Recent developments in voltammetric and amperometric sensors for cysteine detection. RSC Adv 11:5411–5425. https://doi.org/10.1039/d0ra07614g
Dadfarnia S, Assadollahi T, Shabani AMH (2007) Speciation and determination of thallium by on-line microcolumn separation/preconcentration by flow injection-flame atomic absorption spectrometry using immobilized oxine as sorbent. J Hazard Mater 148:446–452. https://doi.org/10.1016/j.jhazmat.2007.02.059
Dourado AHB, Queiroz R, Temperini MLA, Sumodjo PTA (2016) Investigation of the electrochemical behavior of l-cysteine in acidic media. J Electroanal Chem 765:87–91. https://doi.org/10.1016/j.jelechem.2015.09.004
Wang FT, Wang LN, Xu J et al (2021) Synthesis and modification of carbon dots for advanced biosensing application. Analyst 146:4418–4435. https://doi.org/10.1039/d1an00466b
Arcudi F, Ðorđević L, Rebeccani S et al (2021) Lighting up the electrochemiluminescence of carbon dots through pre- and post-synthetic design. Adv Sci 8:1–9. https://doi.org/10.1002/advs.202100125
Zhu S, Meng Q, Wang L et al (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chemie - Int Ed 52:3953–3957. https://doi.org/10.1002/anie.201300519
Atchudan R, Edison TNJI, Lee YR (2016) Nitrogen-doped carbon dots originating from unripe peach for fluorescent bioimaging and electrocatalytic oxygen reduction reaction. J Colloid Interface Sci 482:8–18. https://doi.org/10.1016/j. jcis.2016.07.058
Barhum H, Alon T, Attrash M et al (2021) Multicolor phenylenediamine carbon dots for metal-ion detection with picomolar sensitivity. ACS Appl Nano Mater 4(9):9919–9931. https://doi.org/10.1021/acsanm.1c02496
Zhou Y, Zhang JF, Yoon J (2014) Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem Rev 114:5511–5571. https://doi.org/10.1021/cr4003
Bruno F, Sciortino A, Buscarino G et al (2021) A comparative study of top-down and bottom-up carbon nanodots and their interaction with mercury ions. Nanomaterials 11:1265. https://doi.org/10.3390/nano11051265
Gao D, Zhang Y, Liu A et al (2020) Photoluminescence-tunable carbon dots from synergy effect of sulfur doping and water engineering. Chem Eng J 388:124199. https://doi.org/10.1016/j.cej.2020.124199
Li M, Hu C, Yu C et al (2015) Organic amine-grafted carbon quantum dots with tailored surface and enhanced photoluminescence properties. Carbon 91:291–297. https://doi.org/10.1016/j.carbon.2015.04.083
Li F, Yang D, Xu H (2019) Non-metal-heteroatom-doped carbon dots: synthesis and properties. Chem - A Eur J 25:1165–1176. https://doi.org/10.1002/chem.201802793
Liu Z, Gong Y, Fan Z (2016) Cysteine detection using a high-fluorescence sensor based on a nitrogen-doped graphene quantum dot-mercury(II) system. J Lumin 175:129–134. https://doi.org/10.1016/j.jlumin.2016.01.036
Qu S, Zhou D, Li D et al (2016) Toward efficient orange emissive carbon nanodots through conjugated sp2-Domain controlling and surface charges engineering. Adv Mater 28:3516–3521. https://doi.org/10.1002/adma.201504891
Langer M, Paloncýová M, Medve M et al (2021) Progress and challenges in understanding of photoluminescence properties of carbon dots based on theoretical computations. Appl Mater Today 22:100924. https://doi.org/10.1016/j.apmt.2020.100924
Jin SH, Kim DH, Jun GH et al (2013) Tuning the photoluminescence of graphene quantum dots through the charge transfer effect of functional groups. ACS Nano 7:1239–1245. https://doi.org/10.1021/nn304675g
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, Boston
PavIa DL, Lampman GM, Kriz GS, Vyvyan JR (2008) Introduction to Spectroscopy, 4th edn. Cengage Learning, Boston
Ding H, Yu SB, Wei JS, Xiong HM (2016) Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 10:484–491. https://doi.org/10.1021/acsnano.5b05406
Singaravelu CM, Deschanels X, Rey C, Causse J (2021) Solid-state fluorescent carbon dots for fluorimetric sensing of Hg2+. ACS Appl Nano Mater 4:6386–6397. https://doi.org/10.1021/acsanm.1c01400
Jiang L, Ding H, Lu S et al (2020) Photoactivated fluorescence enhancement in F,N-doped carbon dots with piezochromic behavior. Angew Chemie - Int Ed 59:9986–9991. https://doi.org/10.1002/anie.201913800
Dong F, Sun Y, Wu L et al (2012) Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance. Catal Sci Technol 2:1332–1335. https://doi.org/10.1039/c2cy20049j
Mehta VN, Jha S, Singhal RK et al (2014) Preparation of multicolor emitting carbon dots for HeLa cell imaging. New J Chem 38:6152–6160. https://doi.org/10.1039/C4NJ
Hao Y, Li R, Liu Y et al (2023) The on–off-on fluorescence sensor of hollow carbon dots for detecting Hg2+ and ascorbic acid. J Fluoresc 33:459–469. https://doi.org/10.1007/s10895-022-03057-3
Funding
The work reported in the paper has not received any funding.
Author information
Authors and Affiliations
Contributions
D.J.N.: Investigation, Formal analysis, Writing N.V.: Investigation, S.A.J.: Conceptualization, Supervision, M.G.S.: Supervision and Review.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All the authors consent to publishing the paper.
Conflict of Interest
The authors have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nelson, D.J., Vasimalai, N., John, S.A. et al. On-Off-On Fluorometric Detection of Hg(II) and L-Cysteine Using Red Emissive Nitrogen-Doped Carbon Dots for Environmental and Clinical Sample Analysis. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03598-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03598-9