Abstract
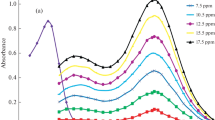
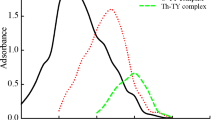
For the determination of tin(II) traces, an extractive spectrophotometric approach is devised. The applied method serves a powerful tool for determination of tin(II), involves the formation of yellow colored complex after the binding of 6-bromo-3-hydroxy-2-(5-methylfuran-2-yl)-4H-chromen-4-one (BHMF) and tin(II) in 1:2 stiochiometry in a slightly acidic medium (HCl). The complex shows absorbance at 434 nm with respect of the blank reagent. The outcomes of spectral investigation for complexation showed a Beer’s range of 0–1.3 μg Sn mL−1, molar absorptivity, specific absorptivity and Sandell’s complex sensitivity are 9.291 × 104 L mol−1 cm−1, 0.490 mL g−1 cm−1 and 0.002040 μg cm−2 at 434 nm that was stable for two days. The interferences study results showed that this method is free from interferences, when tested with metal ions including Ag, Be, Bi, Ca, Cd, Ce, Co, Hg, Mo, Re, Pt, Se,Ti, U, V, W and other common cations, anions, and complexing agents. The applied method is quite simple, highly selective, and sensitive with good re-producibility. This method has been satisfactorily by utilizing the proposed procedure, and its applicability has been tested by analyzing synthetic samples and an alloy sample of gunmetal. The procedure assumes this because of the scarcity of better methods for determining tin(II). The results are in good agreement with the certified value.
Graphical Abstract







Similar content being viewed by others
Availability of Data and Materials
Data and materials are available on demand.
References
Mohan B, Modi K, Patel C, Kumar S, Zhiyu T, You H, Ren P (2021) A new N-methylhydrazinecarbothioamide incorporated “naked-eye” and “turn-off” chemosensor for selective and low detection of Cu2+ ions and computation study. J Photochem Photobiol A Chem 408:113097. https://doi.org/10.1016/j.jphotochem.2020.113097
Gupta SK, Zuniga JP, Pokhrel M, Mao Y (2020) High pressure induced local ordering and tunable luminescence of La2Hf2O7:Eu3+ nanoparticles. New J Chem 44:5463–5472. https://doi.org/10.1039/D0NJ00585A
Saito Y, Nishikawa M, Tsubomura T (2018) Water-soluble copper(I) complexes bearing 2,2′-bicinchoninic acid dipotassium salt with red-light absorption and repeatable colour change upon freezing operation. New J Chem 43:277–283. https://doi.org/10.1039/C8NJ05147J
Kaur B, Kaur N, Kumar S (2018) Colorimetric metal ion sensors – A comprehensive review of the years 2011–2016. Coord Chem Rev 358:13–69. https://doi.org/10.1016/j.ccr.2017.12.002
Saravanan R, Khan MM, Gupta VK, Mosquera E, Gracia F, Narayanan V, Stephen A (2015) ZnO/Ag/CdO nanocomposite for visible light-induced photocatalytic degradation of industrial textile effluents. J Colloid Interface Sci 452:126–133. https://doi.org/10.1016/j.jcis.2015.04.035
Mohan B, Modi K, Patel C, Kumar S, Sharma HK (2020) Synthesis and computational mechanistic studies of copper selective molecular receptor. Vietnam J Chem 58:221–230. https://doi.org/10.1002/vjch.201900161
Machado RC, Amaral CDB, Schiavo D, Nóbrega JA, Nogueira ARA (2017) Complex samples and spectral interferences in ICP-MS: Evaluation of tandem mass spectrometry for interference-free determination of cadmium, tin and platinum group elements. Microchem J 130:271–275. https://doi.org/10.1016/J.MICROC.2016.09.011
Niknezhadi A, Nezamzadeh-Ejhieh A (2017) A novel and sensitive carbon paste electrode with clinoptilolite nano-particles containing hexadecyltrimethyl ammonium surfactant and dithizone for the voltammetric determination of Sn(II). J Colloid Interface Sci 501:321–329. https://doi.org/10.1016/J.JCIS.2017.04.068
Tan KH, Rahman HA, Taib H (2019) Coating layer and influence of transition metal for ferritic stainless steel interconnector solid oxide fuel cell: A review. Int J Hydrogen Energy 44:30591–30605. https://doi.org/10.1016/J.IJHYDENE.2019.06.155
Wang Z, Cheng M, Bu J, Cheng L, Ru J, Hua Y, Wang D (2022) Understanding the electrochemical behavior of Sn(II) in choline chloride-ethylene glycol deep eutectic solvent for tin powders preparation. Adv Powder Technol 33:103670. https://doi.org/10.1016/J.APT.2022.103670
Tan X, Jiang Y, Yiqing C, Anqi T, Li J, Sun Y (2022) Roles of different components of complex inclusion in pitting of 321 stainless steel: Induction effect of CaS and inhibition effect of TiN. Corros Sci 110692. https://doi.org/10.1016/J.CORSCI.2022.110692
Bajnóczi ÉG, Czeglédi E, Kuzmann E, Homonnay Z, Bálint S, Dombi G, Forgo P, Berkesi O, Pálinkó I, Peintler G, Sipos P, Persson I (2014) Speciation and structure of tin(II) in hyper-alkaline aqueous solution. Dalt Trans 43:17971–17979. https://doi.org/10.1039/C4DT02706J
Hu M, Wang G, Zhang Q, Gong J, Xing Z, Gao J, Wang J, Zeng P, Zheng S, Liu M, Zhou Y, Yang S (2022) Antioxidative solution processing yields exceptional Sn(II) stability for sub-1.4 eV bandgap inorganic perovskite solar cells. J Energy Chem 72:487–494. https://doi.org/10.1016/J.JECHEM.2022.05.030
Mohan B, Modi K, Patel C, Bhatia P, Kumar A, Sharma HK (2018) Design and synthesis of two armed molecular receptor for recognition of Gd3+ metal ion and its computational study. Appl Organomet Chem 32:1–11. https://doi.org/10.1002/aoc.4502
Gaur A, Joshi SK, Nair NN, Shrivastava BD, Patel RN, Jha SN, Bhattacharyya D (2020) XAFS study of tridentate Schiff base Ni(II) complexes having distorted octahedral geometry. Radiat Phys Chem 175:108066. https://doi.org/10.1016/J.RADPHYSCHEM.2018.11.020
Pundi A, Chang CJ, Chen J, Hsieh SR, Lee MC (2021) A chiral carbazole based sensor for sequential “on-off-on” fluorescence detection of Fe3+ and tryptophan/histidine. Sens Actuators B Chem 328:129084. https://doi.org/10.1016/j.snb.2020.129084
Likhonina AE, Lebedev IS, Mamardashvili GM, Mamardashvili NZ (2022) pH indicator and rotary fluorescent properties of the Sn(IV)-octaetylporphyrin-(BODIPY)2 triad. Inorganica Chim Acta 542:121150. https://doi.org/10.1016/J.ICA.2022.121150
Singh G, Priyanka, Singh A, Satija P, Sushma, Pawan, Mohit, Singh J, Singh J (2021) Schiff base-functionalized silatrane-based receptor as a potential chemo-sensor for the detection of Al3+ ions. New J Chem 45:7850–7859. https://doi.org/10.1039/d1nj00943e
Singh G, Priyanka, Sushma, Diksha, Mohit, Suman, Kaur JD, Saini A, Satija P (2021) Design, synthesis and photophysical aspects of 1,2,3-triazole appended Schiff base functionalized silanes and silatranes. New J Chem 45:17356–17365. https://doi.org/10.1039/D1NJ03364F
Rahmalia W, Fabre JF, Usman T, Mouloungui Z (2014) Aprotic solvents effect on the UV–visible absorption spectra of bixin. Spectrochim Acta Part A Mol Biomol Spectrosc 131:455–460. https://doi.org/10.1016/J.SAA.2014.03.119
Tolbin AY, Pushkarev VE, Tomilova LG (2018) A mathematical analysis of deviations from linearity of Beer’s law. Chem Phys Lett 706:520–524. https://doi.org/10.1016/J.CPLETT.2018.06.056
Li L, Zhao H, Ni N, Wang Y, Gao J, Gao Q, Zhang Y, Zhang Y (2022) Study on the origin of linear deviation with the Beer-Lambert law in absorption spectroscopy by measuring sulfur dioxide. Spectrochim Acta Part A Mol Biomol Spectrosc 275:121192. https://doi.org/10.1016/J.SAA.2022.121192
Kolcu F, Erdener D, Kaya İ (2020) A Schiff base based on triphenylamine and thiophene moieties as a fluorescent sensor for Cr (III) ions: Synthesis, characterization and fluorescent applications. Inorganica Chim Acta 509:119676. https://doi.org/10.1016/j.ica.2020.119676
Chen H, Yang P, Li Y, Zhang L, Ding F, He X, Shen J (2020) Insight into triphenylamine and coumarin serving as copper (II) sensors with “OFF” strategy and for bio-imaging in living cells. Spectrochim Acta - Part A Mol Biomol Spectrosc 224:117384. https://doi.org/10.1016/j.saa.2019.117384
Kundu S, Truong KN, Saha S, Rissanen K, Sahoo P (2022) A handy and accessible tool for identification of Sn(II) in toothpaste. Sci Rep 12:1–9. https://doi.org/10.1038/s41598-022-06299-0
Gholivand MB, Babakhanian A, Rafiee E (2008) Determination of Sn(II) and Sn(IV) after mixed micelle-mediated cloud point extraction using α-polyoxometalate as a complexing agent by flame atomic absorption spectrometry. Talanta 76:503–508. https://doi.org/10.1016/J.TALANTA.2008.03.057
Acknowledgements
Gratitude to acknowledge the financial assistance provided by Human Resource Development Group (HRDG) Council of Scientific & Industrial Research (CSIR), New Delhi, India, to carry out this work under reference no. 09/105(0212)/2013-EMR-1. The authors are thankful to the Department of chemistry, Kurukshetra University, Kurukshetra (India), for providing spectral facilities, including NMR spectroscopy.
Funding
The financial assistance provided by Human Resource Development Group (HRDG) Council of Scientific & Industrial Research (CSIR), New Delhi, India, to carry out this work under reference no. 09/105(0212)/2013-EMR-1.
Author information
Authors and Affiliations
Contributions
Original draft preparation, writing, methodology by Pankaj Bhatia and Virender. Formal analysis, review and editing by Gurjaspreet Singh. Formal analysis by Harish Kumar Sharma and Brij Mohan.
Corresponding authors
Ethics declarations
Ethical Approval
No such data applies to human and/ or animal studies. This work is novel and not been published till in any journal or thesis work.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhatia, P., Virender, Sharma, H.K. et al. Extractive Spectrophotometric Detection of Sn(II) Using 6-bromo-3-hydroxy-2-(5-methylfuran-2-yl)-4H-chromen-4-one. J Fluoresc (2023). https://doi.org/10.1007/s10895-023-03413-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-023-03413-x