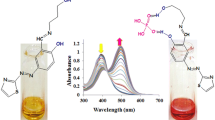
Abstract
A naphthylidene-diimine L2 was newly designed, and its structure was identified by elemental analysis and spectroscopic methods. The effect of temperature, acid–base and light on enol–keto tautomerism in this Schiff base was evaluated by colorimetry, UV–Vis and fluorescence spectroscopy. Under irradiation 365 nm, L2 emitted yellow, orange and strong green emission in pure, basic and aqueous DMSO media (v/v, 1/1), respectively. Its ionochromic behavior against various cations (Fe3+, Al3+, Cr3+, Cu2+, Co2+, Ni2+, Zn2+, Cd2+, Pb2+, Ba2+ and Ag+) and anions (F−, Cl−, CH3COO−, SO32–, S2O32−, HSO4−, H2PO4−, NO3−, CN−, and OH−) was investigated in aqueous DMSO media (v/v, 1/1) by UV–Vis and fluorescence experiments. Dark yellow color of L2 changed to colorless for Fe3+, Cr3+ and HSO4− ions, and turned to light yellow for Al3+ and Cu2+ ions, and to orange for CN− and OH− ions. According to UV–Vis data, the chemosensor displayed selective recognition towards Fe3+, Al3+, Cu2+, HSO4−, CN− and OH− with a 1:1 stoichiometric ratio. At the excitation wavelength of 365 nm, L2 gave strong yellowish white emission (λem = 445 and 539 nm) in the presence of Al3+, and the intensity increased about 12.5 times. On the other hand, the chemosensor displayed one emission band at 452 nm and 450 nm in the presence of CN− and OH− with 1.9 fold and 2.3 fold fluorescence enhancement, respectively.




















Similar content being viewed by others
Availability of Data and Materials
Additional documents are included in supplementary material file. Electronic Supplementary Material associated with this article can be found in the online version of this paper (DOI: xxxxxxxxxx).
References
More MS, Joshi PG, Mishra YK, Khanna PK (2019) Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: a review. Mater Today Chem 14:100195. https://doi.org/10.1016/j.mtchem.2019.100195
Sharbati MT, Rad MNS, Behrouz S, Gharavi A, Emami F (2011) Near infrared organic light-emitting diodes based on acceptor–donor–acceptor (ADA) using novel conjugated isatin Schiff bases. J Lumin 131:553–558. https://doi.org/10.1016/j.jlumin.2010.10.016
Das AK, Goswami S (2017) 2-Hydroxy-1-naphthaldehyde: a versatile building block for the development of sensors in supramolecular chemistry and molecular recognition. Sens Actuat B-Chem 245:1062–1125. https://doi.org/10.1016/j.snb.2017.01.068
Shu J, Ni T, Liu X, Xu B, Liu L, Chu W, Zhang K, Jiang W (2021) Mechanochromism, thermochromism, protonation effect and discrimination of CHCl3 from organic solvents in a Et2N-substituted Salicylaldehyde Schiff base. Dye Pigment 195:109708. https://doi.org/10.1016/j.dyepig.2021.109708
Liu JJ, Fu JJ, Shen X, Liu T, Cheng FX (2022) The effect of dicarboxylic acid isomer on the photochromism of naphthalenediimide-based metal-organic frameworks. J Mol Struct 1265:133346. https://doi.org/10.1016/j.molstruc.2022.133346
Özdemir Güngör Ö (2017) Intramolecular Proton transfer equilibrium in salicylidene- and naphthalene-based Tetraimine Schiff Bases. Gazi U J Sci 30:191–214
Chaihan K, Semakul N, Promarak V, Bui TT, Kungwan N, Goubard F (2022) Tunable far-red fluorescence utilizing π-extension and substitution on the excited state intramolecular proton transfer (ESIPT) of naphthalene-based Schiff bases: a combined experimental and theoretical study. J Photochem Photobiol A 431:114047. https://doi.org/10.1016/j.jphotochem.2022.114047
Mendecki L, Granados-Focil S, Jendrlin M, Mold M, Radu A (2020) Self-plasticized, lumogallion-based fluorescent optical sensor for the determination of aluminium(III) with ultra-low detection limits. Anal Chim Acta 1101:141–148. https://doi.org/10.1016/j.aca.2019.12.021
Qin JC, Cheng XY, Fang R, Wang MF, Yang ZY, Li TR, Li Y (2016) Two Schiff-base fluorescent sensors for selective sensing of aluminum(III): experimental and computational studies. Spectrochim Acta A 152:352–357. https://doi.org/10.1016/j.saa.2015.07.095
Hu T, Wang L, Li J, Zhao Y, Cheng J, Li W, Chang Z, Sun C (2021) A new fluorescent sensor L based on fluorene-naphthalene Schiff base for recognition of Al3+ and Cr3+. Inorg Chim Acta 524:120421. https://doi.org/10.1016/j.ica.2021.120421
Suganya S, Ravindran E, Mahato MK, Prasad E (2019) Orange emitting fluorescence probe for the selective detection of cyanide ion in solution and solid states. Sens Actuat B-Chem 291:426–432. https://doi.org/10.1016/j.snb.2019.04.066
Chakraborty S, Paul S, Roy P, Rayalu S (2021) Detection of cyanide ion by chemosensing and fluorosensing technology. Inorg Chem Comm 128:108562. https://doi.org/10.1016/j.inoche.2021.108562
Sidqi ME, Abdel Aziz AA, Abolehasan AE, Sayed MA (2022) Photochemical processing potential of a novel Schiff base as a fluorescent probe for selective monitoring of Al3+ ions and bioimaging in human cervical cancer HeLa cells. J Photochem Photobiol A 424:113616. https://doi.org/10.1016/j.jphotochem.2021.113616
Guo X, Guo C, Xing Y, Liu Y, Wei K, Kang M, Yang X, Pei M, Zhang G (2022) A novel Schiff base sensor through “off-on-off” fluorescence behavior for sequentially monitoring Al3+ and Cu2+. J Photochem Photobiol A 430:113990. https://doi.org/10.1016/j.jphotochem.2022.113990
Pundi A, Chen J, Chang CJ, Hsieh SR, Lee MC, Chou CH, Way TD (2021) Naked-eye colorimetric and turn-on fluorescent Schiff base sensor for cyanide and aluminum(III) detection in food samples and cell imaging applications. Spectrochim Acta A 262:120139. https://doi.org/10.1016/j.saa.2021.120139
Yilmaz B, Keskinates M, Aydin Z, Bayrakci M (2022) A highly selective optical sensor for the detection of cyanide ions in aqueous solution and living cells. J Photochem Photobiol A 424:113651. https://doi.org/10.1016/j.jphotochem.2021.113651
Wang DF, Ke YC, Guo HX, Chen J, Weng W (2014) A novel highly selective colorimetric sensor for aluminum(III) ion using Schiff base derivative. Spectrochim Acta A 122:268–272. https://doi.org/10.1016/j.saa.2013.11.063
Shoora SK, Jain AK, Gupta VK (2015) A simple Schiff base based novel optical probe for aluminium(III) ions. Sens Actuat B-Chem 216:86–104. https://doi.org/10.1016/j.snb.2015.04.038
Prakash O, Verma RS, Saini RK (1997) Tetrahedron Lett 38:2621
Kumar R, Nair RR, Dhiman SS, Sharma J, Prakash O (2010) Iodine(III)-mediated synthesis of some 2-aryl/ hetarylbenzoxazoles as antibacterial/antifungal agents. Med Chem Res 19:541–550. https://doi.org/10.1007/s00044-009-9211-y
Ozdemir O (2019) Synthesis of new luminescent bis-azo-linkage Schiff bases containing aminophenol and its derivative. Part I: studying of their tautomeric, acidochromic, thermochromic, ionochromic, and photolüminesence properties. J Photochem Photobiol A 380:111868. https://doi.org/10.1016/j.jphotochem.2019.111868
Minkin VI, Tsukanov AV, Dubonosov AD, Bren VA (2011) Tautomeric Schiff bases: Iono-, solvato-, thermo- and photochromism. J Mol Struct 998:179–191. https://doi.org/10.1016/j.molstruc.2011.05.029
Özdemir Ö (2019) Synthesis and characterization of a new diimine Schiff base and its Cu2+ and Fe3+ complexes: investigation of their photoluminescence, conductance, spectrophotometric and sensor behaviors. J Mol Struct 1179:376–389. https://doi.org/10.1016/j.molstruc.2018.11.023
Behura R, Dash PP, Mohanty P, Behera S, Mohanty M, Dinda R, Behera SK, Barick AK, Jali BR (2022) A Schiff base luminescent chemosensor for selective detection of Zn2+ in aqueous medium. J Mol Struct 1264:133310. https://doi.org/10.1016/j.molstruc.2022.133310
Özdemir Ö (2021) A new 2-hydroxynaphthalene based Schiff base receptor for detection of Cu2+, Fe3+, HSO4–, CN– ions and D–amino acids in aqueous DMSO solution. J Mol Struct 1240:130532. https://doi.org/10.1016/j.molstruc.2021.130532
Güngör Ö, Gürkan P (2019) Potentiometric and antimicrobial studies on the asymmetric Schiff bases and their binuclear ni(II) and Fe(III) complexes; synthesis and characterization of the complexes. Arab J Chem 12:2244–2256. https://doi.org/10.1016/j.arabjc.2015.02.009
Özdemir Ö (2018) Studies on phenol-keto tautomerism, metal ion binding, and free radical scavenging properties of newly synthesized naphthalene-based tetraimine Schiff base. J BAUN Inst Sci Technol 20:109–123. https://doi.org/10.25092/baunfbed.412405
Gupta VK, Mergu N, Kumawat LK (2016) A new multifunctional rhodamine-derived probe for colorimetric sensing of Cu(II) and Al(III) and fluorometric sensing of Fe(III) in aqueous media. Sens Actuat B-Chem 223:101–113. https://doi.org/10.1016/j.snb.2015.09.060
Aydın Z, Keleş M (2017) Highly selective Schiff base derivatives for colorimetric detection of Al3+. Turk J Chem 41:89–98. https://doi.org/10.3906/kim-1603-127
El-Kashef HS, Abdel-Hamide R, Mahmoud MR, Hamed MM (1981) Medium effect on acidity constants of some 2-styryl-4-phenylthiazole ethiodide derivatives. Can J Chem 59:731–736
Musikavanhu B, Zhang Y, Zhu D, Xue Z, Yuan R, Wang S, Zhao L (2022) Turn-off detection of cr(III) with chelation enhanced fluorescence quenching effect by a naphthyl hydrazone Shiff base chemosensor. Spectrochim Acta A 281:121599. https://doi.org/10.1016/j.saa.2022.121599
Özdemir Ö (2020) Bis-azo-linkage Schiff bases–Part(II): synthesis, characterization, photoluminescence and DPPH radical scavenging properties of their novel luminescent mononuclear zn(II) complexes. J Photochem Photobiol A 392:112356. https://doi.org/10.1016/j.jphotochem.2020.112356
Lei X, Zou Y, Liang Q, Xu W, Lao S, Yang B, Li L, Yang L, Liu H, Ma LJ (2022) An ultrasensitive 4-(Diethylamino) salicylaldehyd-based fluorescence enhancement probe for the detection of Al3+ in aqueous solutions and its application in cells. J Photochem Photobiol A 428:113854. https://doi.org/10.1016/j.jphotochem.2022.113854
Wan L, Shu Q, Zhu J, Jin S, Li N, Chen X, Chen S (2016) A new multifunctional Schiff-based chemosensor for mask-free fluorimetric and colorimetric sensing of F– and CN–. Talanta 152:39–44. https://doi.org/10.1016/j.talanta.2016.01.045
Kolcu F, Erdener D, Kaya İ (2020) A Schiff base based on triphenylamine and thiophene moieties as a fluorescent sensor for cr(III) ions: synthesis, characterization and fluorescent applications. Inorg Chim Acta 509:119676. https://doi.org/10.1016/j.ica.2020.119676
Bamnavat K, Bhardwaj V, Anand T, Ashok Kumar SK, Sahoo SK (2021) Pyridoxal derived AIEgen as a fluorescent pH sensor. Dye Pigment 184:108844. https://doi.org/10.1016/j.dyepig.2020.108844
Bhardwaj V, Thangaraj A, Varddhan S, Ashok Kumar SK, Crisponi G, Sahoo SK (2020) An aggregation-induced emission active vitamin B6 cofactor derivative: application in pH sensing and detection of latent fingerprints. Photochem Photobiol Sci 19:1402–1409. https://doi.org/10.1039/d0pp00262c
Song I-h, Torawane P, Lee J-S, Warkad SD, Borase A, Sahoo SK, Nimse SB, Kuwar A (2021) The detection of Al3+ and Cu2+ ions using isonicotinohydrazide-based chemosensors and their application to live-cell imaging. Mater Adv 2:6306–6314. https://doi.org/10.1039/d1ma00564b
Gupta VK, Jain AK, Shoora SK (2015) New “on–off” optical probe based on Schiff base respondingto Al3+ions: Logic gate application. Sens Actuat B-Chem 219:218–231. https://doi.org/10.1016/j.snb.2015.05.026
Dey S, Sena C, Sinha C (2020) Chromogenic hydrazide Schiff base reagent: Spectrophotometric determination of CN– ion. Spectrochim Acta A 225:117471. https://doi.org/10.1016/j.saa.2019.117471
Ullah Z, Sonawane PM, Nguyen TS, Garai M, Churchill DG, Yavuz CT (2021) Bisphenol–based cyanide sensing: selectivity, reversibility, facile synthesis, bilateral “OFF-ON” fluorescence, C2ν structural and conformational analysis. Spectrochim Acta A 259:119881. https://doi.org/10.1016/j.saa.2021.119881
Yapar G, Demir N, Kiraz A, Özkat GY, Yıldız M (2022) Synthesis, Biological Activities, antioxidant Properties, and Molecular Docking Studies of Novel Bis-Schiff Base Podands as Responsive Chemosensors for Anions. J Mol Struct 1266:133530. https://doi.org/10.1016/j.molstruc.2022.133530
Wang T, Pang Q, Tong Z, Wang M, Xiao N (2021) Selective sensing of PPi by fluorogenic Al(III)-probe complex in aqueous medium. Spectrochim Acta A 250:119249. https://doi.org/10.1016/j.saa.2020.119249
Zhang M, Gong L, Sun C, Li W, Chang Z, Qi D (2019) A newfluorescent-colorimetric chemosensor based on a Schiff base for detecting Cr3+, Cu2+, Fe3+ and Al3+ ions. Spectrochim Acta A 214:7–13. https://doi.org/10.1016/j.saa.2019.01.089
Azadbakht R, Rashidi S (2014) A new fluorescent chemosensor for Al3+ ion based on schiff base naphthalene derivatives. Spectrochim Acta A 127:329–334. https://doi.org/10.1016/j.saa.2014.02.101
Hu T, Cheng J, Li L, Zhan Y, Li W, Chang Z, Sun C (2019) A new Schiff base fluorescent-colorimetric probe containing fluorenenaphthalene structure: Multifunction detection. Inorg Chim Acta 498:119131. https://doi.org/10.1016/j.ica.2019.119131
Li Q, Zhang JH, Cai Y, Qu WJ, Gao GY, Lin Q, Yao H, Zhang YM, Wei TB (2015) A facile colorimetric and fluorescent cyanide chemosensor: utilization of the nucleophilic addition induced by resonance-assisted hydrogen bond. Tetrahedron 71:857–862. https://doi.org/10.1016/j.tet.2014.12.047
Alreja P, Kau N (2019) Test kit” of chromogenic and ratiometric 1,10-phenanthroline based chemosensor for the recognition of F– and CN– ions. Inorg Chem Comm 110:107600. https://doi.org/10.1016/j.inoche.2019.107600
Mohanty P, Behura R, Bhardwaj V, Dash PP, Sahoo SK, Jali BR (2022) Recent advancement on chromo-fluorogenic sensing of aluminum(III) with Schiff bases. Trends Environ Anal Chem 34:e00166. https://doi.org/10.1016/j.teac.2022.e00166
Bhardwaj V, Bhardwaj K, Sahoo SK (2023) AIE + ESIPT’ active 2–hydroxy–naphthalene hydrazone for the fluorescence turn–on sensing of Al3+. J Fluoresc 33:1157–1164. https://doi.org/10.1007/s10895-022-03138-3
Acknowledgements
We are thankful to Prof. Dr. Metin GÜRÜ, Prof. Dr. Fatih Akkurt and Prof. Dr. Atilla Murathan for providing the necessary facilities of studing with fluorescence spectrophotometer.
Author information
Authors and Affiliations
Contributions
Ö.G. conducted the main research experiments. Ö.G. and L.N. performed luminescence measurements. L.N. drew the graphs. Ö.G. wrote the draft of the manuscript. L.N. agreed the draft of this manuscript.
Corresponding author
Ethics declarations
Declarations
Ethical Approval
The authors declare that this manuscript does not contain research involving human participants and/or animals.
Conflict of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Additional information
This work is dedicated to 100th anniversary of Republic of Turkey.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Güngör, Ö., Nuralin, L. A Novel Naphthylidene-diimine Chemosensor for Selective Colorimetric and Fluorometric Detection of Al3+ and CN− Ions. J Fluoresc 34, 1319–1342 (2024). https://doi.org/10.1007/s10895-023-03368-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03368-z