Abstract
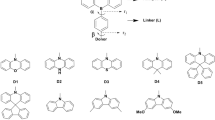
Thermally activated delayed fluorescent emitters based on carbazole donor, benzonitrile acceptor with the linkers biphenyl, bipyridine and naphthalene are investigated using the density functional theoretical method. The molecule in which bipyridine acts as the linker with the least ΔEST is further selected for the designing of a series of D-L-A framework TADF molecules. Remarkably, the ΔEST is decreased successively by attaching the additional cyano groups at the acceptor site which is further reduced when the electron donating methoxy groups are attached at the donor site. To know the effect of substituents on ΔEST, the acceptor moiety of the D-L-A framework is modified with -F, -Cl and -CF3 substituents. The studies showed a relatively less decrement in the value of ΔEST compared to the cyano substituted molecules. However, ΔEST significantly reduced further on attaching methoxy groups at the donor site.



Similar content being viewed by others
Availability of Data and Materials
The coordinates of the optimized geometries are available on request.
References
Zou S-J, Shen Y, Xie F-M et al (2020) Recent advances in organic light-emitting diodes: toward smart lighting and displays. Mater Chem Front 4:788–820
Wei Q, Pötzsch R, Liu X et al (2016) Hyperbranched Polymers with High Transparency and Inherent High Refractive Index for Application in Organic Light-Emitting Diodes. Adv Funct Mater 26:2545–2553
Kafafi ZH, Martín-Palma RJ, Nogueira AF et al (2015) The role of photonics in energy. J Photonics Energy 5:050997
Jadoun S, Riaz U (2020) Conjugated Polymer Light-Emitting Diodes. In: Polymers for Light-Emitting Devices and Displays. John Wiley & Sons, Ltd, 77–98
Hung LS, Chen CH (2002) Recent progress of molecular organic electroluminescent materials and devices. Mater Sci Eng R Rep 39:143–222
Baldo MA, O’Brien DF, Thompson ME, Forrest SR (1999) Excitonic singlet-triplet ratio in a semiconducting organic thin film. Phys Rev B 60:14422–14428
Baldo MA, O’Brien DF, You Y et al (1998) Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395:151–154
Baldo MA, Adachi C, Forrest SR (2000) Transient analysis of organic electrophosphorescence. II. Transient analysis of triplet-triplet annihilation. Phys Rev B 62:10967–10977
Zhang Q, Li B, Huang S et al (2014) Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat Photonics 8:326–332
Pan K-C, Li S-W, Ho Y-Y et al (2016) Efficient and Tunable Thermally Activated Delayed Fluorescence Emitters Having Orientation-Adjustable CN-Substituted Pyridine and Pyrimidine Acceptor Units. Adv Funct Mater 26:7560–7571
Lin T-A, Chatterjee T, Tsai W-L et al (2016) Sky-Blue Organic Light Emitting Diode with 37% External Quantum Efficiency Using Thermally Activated Delayed Fluorescence from Spiroacridine-Triazine Hybrid. Adv Mater 28:6976–6983
Zhang Y-L, Ran Q, Wang Q et al (2019) High-Efficiency Red Organic Light-Emitting Diodes with External Quantum Efficiency Close to 30% Based on a Novel Thermally Activated Delayed Fluorescence Emitter. Adv Mater 31:1902368
Klessinger M (1995) Conical Intersections and the Mechanism of Singlet Photoreactions. Angew Chem Int Ed Engl 34:549–551
Méhes G, Nomura H, Zhang Q et al (2012) Enhanced Electroluminescence Efficiency in a Spiro-Acridine Derivative through Thermally Activated Delayed Fluorescence. Angew Chem Int Ed 51:11311–11315
Busmann H, Staerk H, Weller A (1989) Solvent influence on the magnetic field effect of polymethylene-linked photogenerated radical ion pairs. J Chem Phys 91:4098–4105
Fan J, Lin L, Wang C-K (2017) Excited state properties of non-doped thermally activated delayed fluorescence emitters with aggregation-induced emission: a QM/MM study. J Mater Chem C 5:8390–8399. https://doi.org/10.1039/C7TC02541F
Yang Z, Mao Z, Xie Z et al (2017) Recent advances in organic thermally activated delayed fluorescence materials. Chem Soc Rev 46:915–1016
Ganesan P, Ranganathan R, Chi Y et al (2017) Functional Pyrimidine-Based Thermally Activated Delay Fluorescence Emitters: Photophysics, Mechanochromism, and Fabrication of Organic Light-Emitting Diodes. Chem Eur J 23:2858–2866
Fan J, Lin L, Wang C (2016) Decreasing the singlet–triplet gap for thermally activated delayed fluorescence molecules by structural modification on the donor fragment: First-principles study. Chem Phys Lett 652:16–21
Higginbotham HF, Yi C-L, Monkman AP, Wong K-T (2018) Effects of Ortho-Phenyl Substitution on the rISC Rate of D-A Type TADF Molecules. J Phys Chem C 122:7627–7634
Paras, Dhiman A, Ramachandran CN (2022) Effect of aromatic linkers on thermally activated delayed fluorescence of selected organic molecules. Chem Phys Lett 139711
Gao Y, Su T, Wu Y et al (2016) Triptycences as thermally activated delayed fluorescence materials: Effect of π-conjugation length and donors. Chem Phys Lett 666:7–12
Godumala M, Choi S, Cho MJ, Choi DH (2016) Thermally activated delayed fluorescence blue dopants and hosts: from the design strategy to organic light-emitting diode applications. J Mater Chem C 4:11355–11381
Tanaka H, Shizu K, Miyazaki H, Adachi C (2012) Efficient green thermally activated delayed fluorescence (TADF) from a phenoxazine–triphenyltriazine (PXZ–TRZ) derivative. Chem Commun 48:11392–11394
Im Y, Lee JY (2017) Recent progress of green thermally activated delayed fluorescent emitters. J Inf Disp 18:101–117
Kim JH, Yun JH, Lee JY (2018) Recent Progress of Highly Efficient Red and Near-Infrared Thermally Activated Delayed Fluorescent Emitters. Adv Opt Mater 6:1800255
He Y, Zhou D, Zhang C et al (2022) Orange-red and saturated red thermally activated delayed fluorescent dendrimers for non-doped solution-processed OLEDs. Dyes Pigments 203:110385
Liu H, Zhang K, Zou H et al (2022) A theoretical perspective of the relationship between the structures and luminescence properties of red thermally activated delayed fluorescence molecules. Phys Chem Chem Phys 24:17140–17154
Wang J, Lu J, Zhang J (2017) Tuning the electronic and optical properties of diphenylsulphone based thermally activated delayed fluorescent materials via structural modification: A theoretical study. Dyes Pigments 143:42–47
Zhang K, Yang F, Zhang Y et al (2021) Highly Efficient Near-Infrared Thermally Activated Delayed Fluorescence Molecules via Acceptor Tuning: Theoretical Molecular Design and Experimental Verification. J Phys Chem Lett 12:1893–1903
Wu S, Aonuma M, Zhang Q et al (2013) High-efficiency deep-blue organic light-emitting diodes based on a thermally activated delayed fluorescence emitter. J Mater Chem C 2:421–424
Zhang Q, Li J, Shizu K et al (2012) Design of Efficient Thermally Activated Delayed Fluorescence Materials for Pure Blue Organic Light Emitting Diodes. J Am Chem Soc 134:14706–14709
Wu K, Zhang T, Zhan L, et al (2016) Optimizing Optoelectronic Properties of Pyrimidine-Based TADF Emitters by Changing the Substituent for Organic Light-Emitting Diodes with External Quantum Efficiency Close to 25 % and Slow Efficiency Roll-Off. Chem Eur J 22:10860–10866
Zeng W, Lai H-Y, Lee W-K et al (2018) Achieving Nearly 30% External Quantum Efficiency for Orange-Red Organic Light Emitting Diodes by Employing Thermally Activated Delayed Fluorescence Emitters Composed of 1,8-Naphthalimide-Acridine Hybrids. Adv Mater 30:1704961
Yuan Y, Hu Y, Zhang Y-X et al (2017) Over 10% EQE Near-Infrared Electroluminescence Based on a Thermally Activated Delayed Fluorescence Emitter. Adv Funct Mater 27:1700986
Okazaki M, Takeda Y, Data P et al (2017) Thermally activated delayed fluorescent phenothiazine–dibenzo [a, j]phenazine–phenothiazine triads exhibiting tricolor-changing mechanochromic luminescence. Chem Sci 8:2677–2686
Tsiko U, Bezvikonnyi O, Volyniuk D et al (2022) TADF quenching properties of phenothiazine or phenoxazine-substituted benzanthrones emitting in deep-red/near-infrared region towards oxygen sensing. Dyes Pigments 197:109952
Uoyama H, Goushi K, Shizu K et al (2012) Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492:234–238
Cho YJ, Yook KS, Lee JY (2015) Cool and warm hybrid white organic light-emitting diode with blue delayed fluorescent emitter both as blue emitter and triplet host. Sci Rep 5:7859
Rajamalli P, Senthilkumar N, Gandeepan P et al (2016) A New Molecular Design Based on Thermally Activated Delayed Fluorescence for Highly Efficient Organic Light Emitting Diodes. J Am Chem Soc 138:628–634
Vikramaditya T, Saisudhakar M, Sumithra K (2016) Computational study on thermally activated delayed fluorescence of donor–linker–acceptor network molecules. RSC Adv 6:37203–37211
Penfold TJ (2015) On Predicting the Excited-State Properties of Thermally Activated Delayed Fluorescence Emitters. J Phys Chem C 119:13535–13544
Lu J, Zheng Y, Zhang J (2015) Rational design of phenoxazine-based donor–acceptor–donor thermally activated delayed fluorescent molecules with high performance. Phys Chem Chem Phys 17:20014–20020
Gibson J, J. Penfold T, (2017) Nonadiabatic coupling reduces the activation energy in thermally activated delayed fluorescence. Phys Chem Chem Phys 19:8428–8434
Rupakheti C, Al-Saadon R, Zhang Y et al (2016) Diverse Optimal Molecular Libraries for Organic Light-Emitting Diodes. J Chem Theory Comput 12:1942–1952
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA, Peralta JE Jr., Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2009) Gaussian, Inc., Wallingford CT
Lu T, Chen F (2012) Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Ruiz-Morales Y, Miranda-Olvera AD, Portales-Martínez B, Domínguez JM (2019) Determination of 13C NMR Chemical Shift Structural Ranges for Polycyclic Aromatic Hydrocarbons (PAHs) and PAHs in Asphaltenes: An Experimental and Theoretical Density Functional Theory Study. Energy Fuels 33:7950–7970
CHESHIRE Chemical Shift Repository. http://cheshirenmr.info/. Accessed 8 Jul 2023
Guido CA, Cortona P, Mennucci B, Adamo C (2013) On the Metric of Charge Transfer Molecular Excitations: A Simple Chemical Descriptor. J Chem Theory Comput 9:3118–3126
Li Y, Zou L-Y, Ren A-M, Feng J-K (2012) Theoretical study on the electronic structures and photophysical properties of a series of dithienylbenzothiazole derivatives. Comput Theor Chem 981:14–24
Acknowledgements
Paras acknowledges Council of Scientific and Industrial Research (CSIR), New Delhi for the fellowship. CNR is grateful to the Scientific and Engineering Research Board (SERB) for the Research Grant EMR/2016/008024. The authors acknowledge Indian Institute Technology Roorkee for the infrastructure.
Funding
CNR is grateful to the Scientific and Engineering Research Board (SERB) for the Research Grant EMR/2016/008024.
Author information
Authors and Affiliations
Contributions
CNR conceived the investigation. Paras performed the calculations. Both authors analyzed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not Applicable.
Competing Interests
The authors have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paras, Ramachandran, C.N. Tuning of the Singlet–Triplet Energy Gap of Donor-Linker-Acceptor Based Thermally Activated Delayed Fluorescent Emitters. J Fluoresc 34, 1343–1351 (2024). https://doi.org/10.1007/s10895-023-03365-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03365-2