Abstract
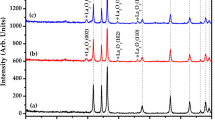
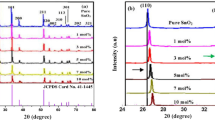
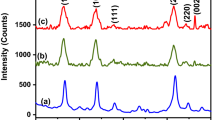
Luminescent antimony doped tin oxide nanoparticles have drawn tremendous attention from researchers due to its low cost, chemical inertness and stability. Herein, a quick, facile and economic hydrothermal/solvothermal method was utilized for the preparation of antimony doped (1%, 3%, 5%, 7% and 10%) tin oxide nanoparticles. The antimony doping in a reasonable range can change the properties of SnO2. As such, a lattice distortion increases with increase in doping, which is evidenced through crystallographic studies. It was found that the highest photocatalytic degradation efficiency of malachite green (MG) dye of about 80.86% was achieved with 10% Sb-doped SnO2 in aqueous media due to small particle size. Moreover, 10% Sb-doped SnO2 also showed the highest fluorescence quenching efficiency of about 27% for Cd2+ of concentration 0.11 µg/ml in the drinking water. The limit of detection (LOD) comes out as 0.0152 µg/ml. This sample selectively detected the cadmium ion even in the presence of other heavy metal ions. Notably, 10% Sb-doped SnO2 could appeared as a promising sensor for fast analysis of Cd2+ ions in real samples.
Graphical Abstract
















Similar content being viewed by others
Data Availability
Data and material information is provided and will be shared on request.
References
Rubin HN, Reynolds MM (2019) Amino-incorporated tricarboxylate metal–organic framework for the sensitive fluorescence detection of heavy metal ions with insights into the origin of photoluminescence response. Inorg Chem 58(16):10671–10679
Kaur H, Bhatti HS, Singh K (2020) Pr doped SnO2 nanostructures: morphology evolution, efficient photocatalysts and fluorescent sensors for the detection of Cd2 + ions in water. J Photochem Photobiol A 388:112144
Zhu YF, Wang YS, Zhou B, Yu JH, Peng LL, Huang YQ, Wang XF (2017) A multifunctional fluorescent aptamer probe for highly sensitive and selective detection of cadmium (II). Anal Bioanal Chem 409(21):4951–4958
Ding SB, Wang W, Qiu LG, Yuan YP, Peng FM, Jiang X, Zhu JF (2011) Surfactant-assisted synthesis of lanthanide metal-organic framework nanorods and their fluorescence sensing of nitroaromatic explosives. Mater Lett 65(9):1385–1387
George G, Edwards CS, Hayes JI, Yu L, Ede SR, Wen J, Luo Z (2019) A novel reversible fluorescent probe for the highly sensitive detection of nitro and peroxide organic explosives using electrospunBaWO 4 nanofibers. J Mater Chem C 7(47):14949–14961
Håkansson K, Coorey RV, Zubarev RA, Talrose VL, Håkansson P (2000) Low-mass ions observed in plasma desorption mass spectrometry of high explosives. J Mass Spectrom 35(3):337–346
Akpan UG, Hameed BH (2009) Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J Hazard Mater 170(2–3):520–529
Borker P, Salker AV (2006) Photocatalytic degradation of textile azo dye over Ce1 – xSnxO2 series. Mater Sci Eng B 133(1–3):55–60
Ahmed AS, Singla ML, Tabassum S, Naqvi AH, Azam A (2011) Band gap narrowing and fluorescence properties of nickel doped SnO2 nanoparticles. J Lumin 131(1):1–6
Bouras K, Rehspringer JL, Schmerber G, Rinnert H, Colis S, Ferblantier G, Slaoui A (2014) Optical and structural properties of nd doped SnO2 powder fabricated by the sol–gel method. J Mater Chem C 2(39):8235–8243
Kolesnikov IE, Kolokolov DS, Kurochkin MA, Voznesenskiy MA, Osmolowsky MG, Lähderanta E, Osmolovskaya OM (2020) Morphology and doping concentration effect on the luminescence properties of SnO2: Eu3+ nanoparticles. J Alloys Compd 822:153640
Bak T, Nowotny J, Rekas M, Sorrell CC (2002) Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int J Hydrog Energy 27(10):991–1022
Kamat PV (2002) Photoinduced transformations in semiconductormetal nanocomposite assemblies. Pure Appl Chem 74(9):1693–1706
Huang Y, Li G, Feng J, Zhang Q (2010) Investigation on structural, electrical and optical properties of tungsten-doped tin oxide thin films. Thin Solid Films 518(8):1892–1896
Batzill M, Diebold U (2005) The surface and materials science of tin oxide. Prog Surf Sci 79(2–4):47–154
Senthilkumar V, Senthil K, Vickraman P (2012) Microstructural, electrical and optical properties of indium tin oxide (ITO) nanoparticles synthesized by co-precipitation method. Mater Res Bull 47(4):1051–1056
Banyamin ZY, Kelly PJ, West G, Boardman J (2014) Electrical and optical properties of fluorine doped tin oxide thin films prepared by magnetron sputtering. Coatings 4(4):732–746
Obaida M, Moussa I, Boshta M (2015) Low sheet Resistance FDoped SnO2 Thin Films deposited by novel spray pyrolysis technique. Int J ChemTech Res 8:239–247
Giraldi TR, Escote MT, Maciel AP, Longo E, Leite ER, Varela JA (2006) Transport and sensors properties of nanostructured antimony-doped tin oxide films. Thin Solid Films 515(4):2678–2685
Senguttuvan TD, Malhotra LK (1996) Sol gel deposition of pure and antimony doped tin dioxide thin films by non alkoxide precursors. Thin Solid Films 289(1–2):22–28
Jain G, Kumar R (2004) Electrical and optical properties of tin oxide and antimony doped tin oxide films. Opt Mater 26(1):27–31
Babar AR, Shinde SS, Moholkar AV, Bhosale CH, Kim JH, Rajpure KY (2011) Physical properties of sprayed antimony doped tin oxide thin films: the role of thickness. J Semicond 32(5):053001
Bhat JS, Maddani KI, Karguppikar AM (2006) Influence of Zn doping on electrical and optical properties of multilayered tin oxide thin films. Bull Mater Sci 29(3):331–337
Vijayalakshmi S, Venkataraj S, Subramanian M, Jayavel R (2008) Physical properties of zinc doped tin oxide films prepared by spray pyrolysis technique. J Phys D 41(3):035505
Al-Hamdi AM, Sillanpää M, Bora T, Dutta J (2016) Efficient photocatalytic degradation of phenol in aqueous solution by SnO2: Sb nanoparticles. Appl Surf Sci 370:229–236
Tayade RJ, Natarajan TS, Bajaj HC (2009) Photocatalytic degradation of methylene blue dye using ultraviolet light emitting diodes. Ind Eng Chem Res 48(23):10262–10267
Ahmed MA, El-Katori EE, Gharni ZH (2013) Photocatalytic degradation of methylene blue dye using Fe2O3/TiO2 nanoparticles prepared by sol–gel method. J Alloys Compd 553:19–29
Sujatha K, Seethalakshmi T, Sudha AP, Shanmugasundaram OL (2020) Photoluminescence properties of pure, Fe-doped and surfactant-assisted Fe-doped tin-oxide nanoparticles. Bull Mater Sci 43(1):1–10
Qu X, Wang MH, Chen Y, Sun WJ, Yang R, Zhang HP (2017) Facile synthesis of hierarchical SnO2 twig-like microstructures and their applications in humidity sensors. Mater Lett 186:182–185
Ansari SG, Boroojerdian P, Sainkar SR, Karekar RN, Aiyer RC, Kulkarni SK (1997) Grain size effects on H2 gas sensitivity of thick film resistor using SnO2 nanoparticles. Thin Solid Films 295(1–2):271–276
Singh G, Kohli N, Singh RC (2017) Preparation and characterization of Eu-doped SnO 2 nanostructures for hydrogen gas sensing. J Mater Sci Mater Electron 28:2257–2266
Chen W, Zhou Q, Wan F, Gao T (2012) Gas sensing properties and mechanism of nano-SnO2-based sensor for hydrogen and carbon monoxide. J Nanomater 2012:1–1
Weber IT, Valentini A, Probst LFD, Longo E, Leite ER (2004) Influence of noble metals on the structural and catalytic properties of Ce-doped SnO2 systems. Sens Actuators B 97(1):31–38
Vegard L (1921) Die konstitution der mischkristalle und die raumfüllung der atome. Z Physik 5(1):17–26
Pearson GL, Bardeen J (1949) Electrical properties of pure silicon and silicon alloys containing boron and phosphorus. Phys Rev 75(5):865
Acknowledgements
One of the authors, Deepika Garg is grateful to University Grants Commission (UGC), New Delhi, India, for providing senior research fellowship. Heena, one of another is also thankful to the UGC, New Delhi, India, for providing financial support under College for Potential and Excellence research grant with reference number KCP/2020/MS/2174–2179.
Funding
The authors did not receive support from any organization for the submitted work and have no financial or non- financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Deepika Garg: Performed the experimentation. Gurdeep Singh, Heena Rekhi, Manpreet Kaur, Rajpal Verma: Helped in performing the experiments. Karamjit Singh and Ashok Kumar Malik: Helped in writing and supervised the research work.
Corresponding author
Ethics declarations
Ethics Approval
There are no ethic approvals required for this research work.
Consent to Participate
All authors will participate in the revision of the manuscript.
Consent for publication
All authors agree for the publication.
Conflicts of Interest/Competing Interests
All the authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garg, D., Singh, G., Rekhi, H. et al. Pure and Antimony-doped Tin Oxide Nanoparticles for Fluorescence Sensing and Dye Degradation Applications. J Fluoresc 34, 449–463 (2024). https://doi.org/10.1007/s10895-023-03283-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03283-3