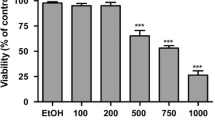
Abstract
Despite the initial success of chemotherapy in the treatment of colorectal carcinoma (CRC), the recurrence of the disease shows that the tumor response is limited by the formation of drug resistance and cannot be kept under control. These drawbacks are associated with the cytostatic chemotherapeutic agent cisplatin (Cis). Combination treatment with different anticancer drugs could increase the therapeutic efficacy of combined therapies by allowing the use of lower, less toxic doses to achieve more efficient destruction of cancer cells. Luteolin (LU) has been studied with other anticancer drugs due to its anticancer cell inhibitory properties and has been shown to sensitize the cytotoxicity induced by various anticancer drugs in several cancer cells. Therefore, in this work, the CompuSyn system was used to investigate different Cis + LU combinations in HCT116 colorectal cancer cells. Immunofluorescence was used to measure mitochondrial membrane potential (MMP) and cell death. As a result, a synergistic effect was observed in 5 of the 7 doses tested. Apoptosis/necrosis resulting from chemotherapy resistance was confirmed by Hoechst/ PI -double staining and mitochondrial dysfunction were determined by Rodamine123 (Rho123). Luteolin could thus be used in medicine to provide more effective cancer therapy in appropriate doses, which promises a promising future in clinical application.





Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Lin Y, Shi R, Wang X, Shen HM (2008) Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets 8(7):634–646
López-Lázaro M (2009) Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem 9(1):31–59
Ginwala R, Bhavsar R, Chigbu DI, Jain P, Khan ZK (2019) Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 8(2):35
Karak P (2019) Biological activities of flavonoids: an overview. Int J Pharm Sci Res 10(4):1567–1574
Karthika C, Sureshkumar R (2021) Incorporation of natural assumption to deal with cancer. Environ Sci Pollut Res Int 28(5):4902–4917
Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA et al (2019) Luteolin, a flavonoid, as an anticancer agent: A review. Biomed Pharmacother 112:108612
Pandurangan AK, Esa NM (2014) Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: a review. Asian Pac J Cancer Prev 15(14):5501–5508
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378
Liu YP, Zheng CC, Huang YN, He ML, Xu WW, Li B (2021) Molecular mechanisms of chemo-and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2(3):315–340
Aldossary SA (2019) Review on pharmacology of cisplatin: clinical use, toxicity and mechanism of resistance of cisplatin. Biomed Pharmacol J 12(1):7–15
Yimit A, Adebali O, Sancar A, Jiang Y (2019) Differential damage and repair of DNA-adducts induced by anti-cancer drug cisplatin across mouse organs. Nat Commun 10(1):1–11
Tang C, Livingston MJ, Safirstein R, Dong Z (2022) Cisplatin nephrotoxicity: new insights and therapeutic implications. Nat Rev Nephrol 1–20
Brito AF, Ribeiro M, Abrantes AM, Pires AS, Teixo RJ, Tralhão JG et al (2015) Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Curr Med Chem 22(26):3025–3039
Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B et al (2017) Combination therapy in combating cancer. Oncotarget 8(23):38022–38043
Rejhová A, Opattová A, Čumová A, Slíva D, Vodička P (2018) Natural compounds and combination therapy in colorectal cancer treatment. Eur J Med Chem 144:582–594
Chu M, Zheng C, Chen C, Song G, Hu X, Wang ZW (2022) Targeting cancer stem cells by nutraceuticals for cancer therapy. Semin Cancer Biol 85:234–245
Soltanian S, Riahirad H, Pabarja A, Jafari E, Khandani BK (2018) Effect of Cinnamic acid and FOLFOX in diminishing side population and downregulating cancer stem cell markers in colon cancer cell line HT-29. Daru 26(1):19–29
Golshani G, Zhang Y (2020) Advances in immunotherapy for colorectal cancer: a review. Therap Adv Gastroenterol 13:1756284820917527
Andreotti PE, Cree IA, Kurbacher CM, Hartmann DM, Linder D, Harel G et al (1995) Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res 55(22):5276–5282
Hunter EM, Sutherland LA, Cree IA, Subedi AM, Hartmann D, Linder D et al (1994) The influence of storage on cytotoxic drug activity in an ATP-based chemosensitivity assay. Anticancer Drugs 5(2):171–176
Kurbacher CM, Cree IA, Bruckner HW, Brenne U, Kurbacher JA, Müller K et al (1998) Use of an ex vivo ATP luminescence assay to direct chemotherapy for recurrent ovarian cancer. Anticancer Drugs 9(1):51–57
Orellana EA, Kasinski AL (2016) Sulforhodamine B (SRB) assay in cell culture to investigate cell proliferation. Bio Protoc 6(21):e1984-1984
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70(2):440–446
Crowley LC, Marfell BJ, Waterhouse NJ (2016) Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb Protoc 2016(9):pdb-prot087205
Tchounwou PB, Dasari S, Noubissi FK, Ray P, Kumar S (2021) Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J Exp Pharmacol 13:303
Amable L (2016) Cisplatin resistance and opportunities for precision medicine. Pharmacol Res 106:27–36
Drögemöller BI, Wright GE, Lo C, Le T, Brooks B, Bhavsar AP et al (2019) Pharmacogenomics of cisplatin-induced ototoxicity: successes, shortcomings, and future avenues of research. Clin Pharmacol Ther 106(2):350–359
Kenguva G, Bandaru R, Rout SR, Greish K, Kesharwani P, Dandela R (2022) Combination drug delivery approaches for cancer therapy. In Combination Drug Delivery Approach as an Effective Therapy for Various Diseases (pp. 213–237). Academic Press
Tarannum M, Hossain MA, Holmes B, Yan S, Mukherjee P, Vivero-Escoto JL (2022) Advanced nanoengineering approach for target-specific, spatiotemporal, and ratiometric delivery of gemcitabine-cisplatin combination for improved therapeutic outcome in pancreatic cancer. Small 18(2):2104449
Li J, Xu H, McIndoe RA (2022) A novel network based linear model for prioritization of synergistic drug combinations. PLoS ONE 17(4):e0266382
Chou TC (2006) Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58(3):621–681
Chou TC (2011) The mass-action law based algorithm for cost-effective approach for cancer drug discovery and development. Am J Cancer Res 1(7):925–954
Daker M, Ahmad M, Khoo AS (2012) Quercetin-induced inhibition and synergistic activity with cisplatin–a chemotherapeutic strategy for nasopharyngeal carcinoma cells. Cancer Cell Int 12(1):1–8
Li QC, Liang Y, Hu GR, Tian Y (2016) Enhanced therapeutic efficacy and amelioration of cisplatin-induced nephrotoxicity by quercetin in 1,2-dimethyl hydrazine-induced colon cancer in rats. Indian J Pharmacol 48(2):168–171
Li T, Li Y (2022) Quercetin acts as a novel anti-cancer drug to suppress cancer aggressiveness and cisplatin-resistance in nasopharyngeal carcinoma (NPC) through regulating the Yes-associated protein/Hippo signaling pathway. Immunobiology 152324
Liu H, Lee JI, Ahn TG (2019) Effect of quercetin on the anti-tumor activity of cisplatin in EMT6 breast tumor-bearing mice. Obstet Gynecol Sci 62(4):242–248
Wang H, Luo Y, Qiao T, Wu Z, Huang Z (2018) Luteolin sensitizes the antitumor effect of cisplatin in drug-resistant ovarian cancer via induction of apoptosis and inhibition of cell migration and invasion. J Ovarian Res 11(1):93
Bukowski K, Kciuk M, Kontek R (2020) Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 21(9):3233
Sarosiek KA, Fraser C, Muthalagu N, Bhola PD, Chang W, McBrayer SK et al (2017) Developmental regulation of mitochondrial apoptosis by c-Myc governs age- and tissue-specific sensitivity to Cancer therapeutics. Cancer Cell 31(1):142–156
Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR et al (2007) Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA 104(49):19512–19517
Nazim UM, Park SY (2019) Luteolin sensitizes human liver cancer cells to TRAIL-induced apoptosis via autophagy and JNK-mediated death receptor 5 upregulation. Int J Oncol 54(2):665–672
Chen P, Hu T, Ma Y, Chen X, Dai L, Lei N et al (2015) Abstract 2808: luteolin inhibits cell proliferation and induces cell apoptosis via down-regulation of mitochondrial membrane potential in esophageal carcinoma cells EC1 and KYSE450. Cancer Res 75(15_Supplement):2808
Xavier CP, Lima CF, Preto A, Seruca R, Fernandes-Ferreira M, Pereira-Wilson C (2009) Luteolin, quercetin and ursolic acid are potent inhibitors of proliferation and inducers of apoptosis in both KRAS and BRAF mutated human colorectal cancer cells. Cancer Lett 281(2):162–170
Ulukaya E, Acilan C, Yilmaz Y (2011) Apoptosis: why and how does it occur in biology? Cell Biochem Funct 29(6):468–480
Abate M, Festa A, Falco M, Lombardi A, Luce A, Grimaldi A et al (2020) Mitochondria as playmakers of apoptosis, autophagy, and senescence. In Seminars in cell & developmental biology (Vol. 98, pp. 139–153). Academic Press
Gray MW (2011) The incredible shrinking organelle. EMBO Rep 1 12(9):873
Bruckmueller H, Cascorbi I (2021) ABCB1, ABCG2, ABCC1, ABCC2, and ABCC3 drug transporter polymorphisms and their impact on drug bioavailability: what is our current understanding? Expert Opin Drug Metab Toxicol 17(4):369–396
Chian S, Li YY, Wang XJ, Tang XW (2014) Luteolin sensitizes two oxaliplatin-resistant colorectal cancer cell lines to chemotherapeutic drugs via inhibition of the Nrf2 pathway. Asian Pac J Cancer Prev 15(6):2911–2916
Acknowledgements
I would like to thank Dr. Ferdane Danışman-Kalındemirtaş for his extensive knowledge of the Compusyn approach.
Author information
Authors and Affiliations
Contributions
Concept, design, supervision, sources, data collection and processing, analysis and interpretation, literature review and writing the manuscript is done by D.Ö.
Corresponding author
Ethics declarations
Ethical Approval
HCT116 and BEAS-2B cell lines were obtained from Istanbul University Faculty of Medicine, Physiology Department. Since they are commercially produced cell lines, no ethics committee approval is required.
Consent to Publication
I, the undersigned, give my consent for the publication of identifiable details, which can include photograph(s) and details within the text (“Material”) to be published in the Journal of Fluorescence. I confirm that I have seen and been given the opportunity to read both the Material and the Article (as attached) to be published by Springer.
Consent to Participate
I consent to participate that Journal of Fluorescence will be available in both print and on the internet, and will be available to a broader audience through marketing channels and other third parties. Therefore, anyone can read material published in the Journal. I understand that readers may include not only medical professionals and scholarly researchers but also journalists and general members of the public.
Competing Interests
There is no competing interest.
Conflicts of interest
Authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Özerkan, D. The Determination of Cisplatin and Luteolin Synergistic Effect on Colorectal Cancer Cell Apoptosis and Mitochondrial Dysfunction by Fluorescence Labelling. J Fluoresc 33, 1217–1225 (2023). https://doi.org/10.1007/s10895-023-03145-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03145-y