Abstract
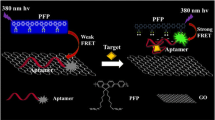
Insulin, the only hormone regulating blood glucose level, is strongly associated with diabetes and its complications. Specific recognition and ultrasensitive detection of insulin are of clinical significance for the early diagnosis and treatment of diabetes. Inspired by aggregation-induced emission, we presented a turn-on label-free fluorescence aptasensor for insulin detection. Quaternized tetraphenylethene salt was synthesized as the fluorescence probe. Guanine-rich aptamer IGA3 was selected as recognition element. Graphene oxide was chosen as the quencher. Under optimized conditions, the fluorescence aptasensor displayed a wide linear range (1.0 pM–1.0 μM) with a low limit of detection (0.42 pM). Furthermore, the aptasensor was successfully applied to detect insulin in human serum. Spiked recoveries were obtained in the range of 96.06%–104.26%. All these results demonstrated that the proposed approach has potential application in the clinical diagnostics of diabetes.







Similar content being viewed by others
Data Availability
All data recorded and analyzed in this article are already included. Data will be available based on reasonable request.
Code Availability
Not applicable.
References
Wu T, Qiao SX, Shi CZ, Wang SY, Ji G (2018) Metabolmics window into diabetic complications. J Diabetes Investig 9:244–255. https://doi.org/10.1111/jdi.12723
Soffe R, Nock V, Chase JG (2019) Towards point-of-care insulin detection. ACS Sens 4:3–19. https://doi.org/10.1021/acssensors.8b01253
Rosli N, Kwon HJ, Lim J, Yoon YA, Jeong JS (2022) Measurement comparability of insulin assays using conventional immunoassay kits. J Clin Lab Anal 36:e24521. https://doi.org/10.1002/jcla.24521
Ho ENM, Wan TSM, Wong ASY, Lam KKH, Stewart BD (2011) Doping control analysis of insulin and its analogues in equine urine by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1218:1139–1146. https://doi.org/10.1016/j.chroma.2010.12.052
Cho H, Kumar S, Yang D, Vaidyanathan S, Woo K, Garcia I, Shue HJ, Yoon Y, Ferreri K, Choo H (2018) Surface-enhanced raman spectroscopy-based label-free insulin detection at physiological concentrations for analysis of islet performance. ACS Sens 3:65–71. https://doi.org/10.1021/acssensors.7b00864
Wu Y, Midinov B, White RJ (2019) Electrochemical aptamer-based sensor for real-time monitoring of insulin. ACS Sens 4:498–503. https://doi.org/10.1021/acssensors.8b01573
Yu GG, Sun Z, Wu YT, Sai N (2022) Dual-QDs ratios fluorescent probe for sensitive and stable detection of insulin. Spectrochim Acta Part A Mol Biomol Spectrosc 268:120641. https://doi.org/10.1016/j.saa.2021.120641
Lian K, Feng HY, Liu SX, Wang KJ, Liu Q, Deng LP, Wang GY, Chen YH, Liu GZ (2022) Insulin quantification towards early diagnosis of prediabetes/diabetes. Biosens Bioelectron 203:114029. https://doi.org/10.1016/j.bios.2022.114029
Shafiei-Irannejad V, Soleymani J, Azizi S, KhoubnasabJafari M, Jouyban A, Hasanzadeh M (2019) Advanced nanomaterials towards biosensing of insulin: Analytical approaches. Trends Anal Chem 116:1–12. https://doi.org/10.1016/j.trac.2019.04.020
Shen YX, Prinyawiwatkul W, Xu ZM (2019) Insulin: a review of analytical methods. Analyst 144:4139–4148. https://doi.org/10.1039/C9AN00112C
Luong AD, Roy I, Malhotra BD, Luong JHT (2021) Analytical and biosensing platforms for insulin: A review. Sen Actuators Rep 000:100028. https://doi.org/10.1016/j.snr.2021.100028
Soffe R, Nock V, Chase JG (2019) Towards point-of-care insulin detection. ACS Sens 49:3–19. https://doi.org/10.1021/acssensors.8b01253
Feng BB, You J, Zhao F, Wei M, Liu Y, Yuan K, Suo ZG (2022) A ratiometric fluorescent aptamer homogeneous biosensor based on hairpin structure aptamer for AFB1 detection. J Fluoresc 32:1695–1701. https://doi.org/10.1007/s10895-022-02972-9
Verdian-Doghaei A, Housaindokht MR (2015) Spectroscopic study of the interaction of insulin and its aptamer - sensitive optical detection of insulin. J Lumin 159:1–8. https://doi.org/10.1016/j.jlumin.2014.10.025
Lu DQ, He L, Zhang G, Lv AP, Wang RW, Zhang XB, Tan WH (2017) Aptamer-assembled nanomaterials for fluorescent sensing and imaging. Nanophotonics 6:109–121. https://doi.org/10.1515/nanoph-2015-0145
Zanchetta G, Lanfranco R, Giavazzi F, Bellini T, Buscaglia M (2017) Emerging applications of label-free optical biosensors. Nanophotonics 6:627–645. https://doi.org/10.1515/nanoph-2016-018
Li DX, Li D, Zong L, Xiao YH, Sui SH, Zhuang B, Li R, Zhen HL, Li J, Huang ZP, Jiang ZG, Wu WH (2022) An AIE fluorescent sensor for rapid and selective detection of phosgene. Chem Commun 58:5296–5299. https://doi.org/10.1039/D2CC00745B
Babu E, Bhuvaneswari J, Mareeswaran PM, Thanasekaran P, Lee HM, Rajagopa S (2019) Transition metal complexes based aptamers as optical diagnostic tools for disease proteins and biomolecules. Coordin Chem Rev 380:519–549. https://doi.org/10.1016/j.ccr.2018.09.010
Lu LH, Wang WH, Wang MD, Kang TS, Lu JJ, Chen XP, Han QB, Leung CH, Ma DL (2016) A luminescent G-quadruplex-selective iridium (III) complex for the label-free detection of lysozyme. J Mater Chem B 4:2407–2411. https://doi.org/10.1039/C6TB00426A
Niu GL, Zhang RY, Shi XJ, Park H, Xie S, Kwok RTK, Lam JWY, Tang BZ (2020) AIE luminogens as fluorescent bioprobes Trends. Anal Chem 123:115769. https://doi.org/10.1016/j.trac.2019.115769
Leung NLC, Xie N, Yuan WZ, Liu Y, Wu QY, Peng Q, Miao Q, Lam JWY, Tang BZ (2014) Restriction of intramolecular motions: The general mechanism behind aggregation-induced emission. Chem Eur J 20:15349–15353. https://doi.org/10.1002/chem.201403811
Wu YP, Shi YC, Deng S, Wu CY, Deng RJ, He GP, Zhou M, Zhong K, Gao H (2021) Metal-induced G-quadruplex polymorphism for ratiometric and label-free detection of lead pollution in tea. Food Chem 343:128425. https://doi.org/10.1016/j.foodchem.2020.128425
Zhang S, Ma L, Ma K, Xu B, Liu LJ, Tian WJ (2018) Label-free aptamer-based biosensor for specific detection of chloramphenicol using AIE probe and graphene oxide. ACS Omega 3:12886–12892. https://doi.org/10.1021/acsomega.8b01812
Li B, Liu CC, Pan WL, Shen JL, Guo JY, Luo TT, Feng JJ, Situ B, An TX, Zhang Y, Zheng L (2020) Facile fluorescent aptasensor using aggregation-induced emission luminogens for exosomal proteins profiling towards liquid biopsy. Biosens Bioelectron 168:112520. https://doi.org/10.1016/j.bios.2020.112520
Duong DL, Sidhanath VB, Lathe AJ, Sheshanath VB (2018) Tetraphenylethylene-based AIE-active probes for sensing applications. ACS Appl Mater Interfaces 10:12189–12216. https://doi.org/10.1021/acsami.7b12320
Yoshida W, Mochizuki E, Takase M, Hasegawa H, Morita Y, Yamazaki H, Sode K, Ikebukuro K (2009) Selection of DNA aptamers against insulin and construction of an aptameric enzyme subunit for insulin sensing. Biosens Bioelectron 24:1116–1120. https://doi.org/10.1016/j.bios.2008.06.016
Ma K, Li X, Xu B, Tian WJ (2021) Label-free bioassay with graphene oxide-based fluorescent aptasensors: A review. Anal Chim Acta 1188:338859. https://doi.org/10.1016/j.aca.2021.338859
Xu JP, Song ZG, Fang Y, Mei J, Jia L, Qin AJ, Sun JZ, Ji J, Tang BZ (2010) Label-free fluorescence detection of mercury (II) and glutathione based on Hg2+-DNA complexes stimulating aggregation-induced emission of a tetraphenylethene derivative. Analyst 135:3002–3007. https://doi.org/10.1039/C0AN00554A
Lou XD, Zhuang Y, Zuo XL, Jia YM, Hong YN, Min XH, Zhang ZY, Xu XM, Liu NN, Xia F, Tang BZ (2015) Real-time, quantitative lighting-up detection of telomerase in urines of bladder cancer patients by AIEgens. Anal Chem 87(13):6822–6827. https://doi.org/10.1021/acs.analchem.5b01099
He YH, Cheng YS, Wen XY (2022) A design of red emission CDs-based aptasensor for sensitive detection of insulin via fluorescence resonance energy transfer. Spectrochim Acta Part A Mol Biomol Spectrosc 280:121497. https://doi.org/10.1016/j.saa.2022.121497
Tan S, Han R, Wu SL, Liang H, Zhao YT, Zhao H, Lia CP (2019) A novel fluorescent sensing platform for insulin detection based on competitive recognition of cationic pillar [6] arene. Talanta 197:130–137. https://doi.org/10.1016/j.talanta.2019.01.004
Muhammad S, Xu GH, Wei FD, Cen Y, Younis MR, Xu XM, Shi ML, Cheng X, Chai YY, Hu Q (2019) Simultaneous determination of insulin and glucose in human serum based on dual emissive fluorescent nano-aptasensor of carbon dots and CdTe/CdS/ ZnS quantum dots. Sens Actuators B Chem 292:321–330. https://doi.org/10.1016/j.snb.2019.04.119
Pourreza N, Ghomi M (2017) A novel metal enhanced fluorescence bio probe for insulin sensing based on Poly vinyl alcohol-borax hydrogel functionalized by Ag dots. Sens Actuators B Chem 251:609–616. https://doi.org/10.1016/j.snb.2017.05.073
Yang JJ, Zhang ZF, Yan GQ (2018) An aptamer-mediated CdSe/ZnS QDs@graphene oxid composite fluorescent probe for specific detection of insulin. Sens Actuators B Chem 255:2339–2346. https://doi.org/10.1016/j.snb.2017.09.046
Wang YH, Gao DY, Zhang PF, Gong P, Chen C, Gao GH, Cai LT (2014) A near infrared fluorescence resonance energy transfer based aptamer biosensor for insulin detection in human plasma. Chem Commun 250:811–813. https://doi.org/10.1039/C3CC47649A
Pu Y, Zhu Z, Han D, Liu HX, Liu J, Liao J, Zhang KJ, Tan WH (2011) Insulin-binding aptamer-conjugated graphene oxide for insulin detection. Analyst 136:4138–4140. https://doi.org/10.1039/C1AN15407A
Funding
This work was supported by the Program for Public Welfare Technology of Zhejiang Province (LGF20B050001), Natural Science Foundation of Zhejiang Province (LY20B050009), and Program for Science and Technology of Jiaxing City (2018AY11002).
Author information
Authors and Affiliations
Contributions
Xiaohui Zeng: investigation, writing-original draft, Hailong Wang: Conceptualization, Supervision, Investigation, Writing—review & editing, Funding acquisition. Yanbo Zeng, Yiwen Yang and Zulei Zhang: Writing—review & editing. Lei Li: Funding acquisition, Resources, Supervision, Writing- review & editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
This article does not contain any studies with human or animal subjects.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, X., Wang, H., Zeng, Y. et al. Label-free Aptasensor for the Ultrasensitive Detection of Insulin Via a Synergistic Fluorescent Turn-on Strategy Based on G-quadruplex and AIEgens. J Fluoresc 33, 955–963 (2023). https://doi.org/10.1007/s10895-022-03116-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03116-9