Abstract
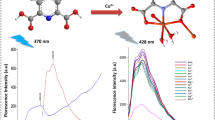
An imidazole possessing sensor (1) has been designed and developed by simple one step reaction and characterization was done by using common spectroscopic methods. The fluorimetric sensing of nerve agent mimic, DCP, was carried out by observing blue shift in spectra accompanied with quenching in semi-aqueous solvent. The sensor was found proficient for the detection of DCP amongst other phosphates with detection limit of 69 nM. Furthermore, upon incorporation of various metal ions to CH3CN:H2O (4:1, v/v) solution of 1 (λex 340 nm), the fluorescent probe turned non-fluorescent only in presence of Cu2+/Hg2+ ions. This was accompanied by fluorescent color change from light blue to yellow in case of Hg2+ and colorless in case of Cu2+ ions. Moreover, practical applications of sensor 1 were investigated for recognition of Cu2+ and Hg2+ ions in real water samples along with the detection of DCP in soil samples from different areas. Differential emission changes observed with addition of Hg2+ ions and DCP led to observation of “NOR” and an “INHIBIT” molecular photonic logic operations at 446 and 385 nm, respectively.










Similar content being viewed by others
Data Availability
The data provided in the manuscript is original and will be made available at any time on request basis.
Code Availability
Not Applicable.
References
Ritchie H, Hasell J, Mathieu E, Appel C, Roser M (2013) Terrorism. https://ourworldindata.org/terrorism (Accessed: 27 Jan 2020)
John H, Worek F, Thiermann H (2008) LC-MS-based procedures for monitoring of toxic organophosphorus compounds and verification of pesticide and nerve agent. Anal Bioanal Chem 391:97–116
Makinen MA, Anttalainen OA, Sillanpaa MET (2010) Ion mobility spectrometry and its applications in detection of chemical warfare agents. Anal Chem 82:9594–9600
Popiel S, Sakowska M (2011) Determination of chemical warfare agents and related compounds in environmental samples by solid-phase microextraction with gas chromatography. J Chromatograph A 1218:8457–8479
Morelato M, Beavis A, Kirkbride P, Roux C (2013) Forensic applications of desorption electrospray ionisation mass spectrometry (DESI-MS). Forensic Sci Int 226:10–21
Giannoukos S, Brkic B, Taylor S, Marshall A, Verbeck GF (2016) Chemical sniffing instrumentation for security applications. Chem Rev 116:8146–8172
Kingery AF, Allen HE (1995) The environmental fate of organophosphorus nerve agents: a review. Toxicol Environ Chem 47:155–184
Diaz de Grenu B, Moreno D, Torroba T, Berg A, Gunnars J, Nilsson T, Nyman R, Persson M, Petterson J, Eklind I, Wasterby P (2014) Fluorescent discrimination between traces of chemical warfare agents and their mimics. J Am Chem Soc 136:4125–4128
Zhou X, Zheng Y, Liyan C, Wu X, Yoon J (2016) A fluorescent sensor for dual-channel discrimination between phosgene and a nerve-gas mimic. Angew Chem Int Ed 55:4729–4733
So HS, Angupillai S, Son YA (2016) Prompt liquid-phase visual detection and low-cost vapor-phase detection of DCP, a chemical warfare agent mimic. Sens Actuators B Chem 235:447–456
Fu Y, Yu J, Wang K, Liu H, Yu Y, Liu A, Peng X, He Q, Cao H, Cheng J (2018) Simple and efficient chromophoric-fluorogenic probes for diethylchlorophosphate vapor. ACS Sens 3:1445–1450
Zeng L, Zeng H, Jiang L, Wang S, Hou JT, Yoon J (2019) A single fluorescent chemosensor for simultaneous discriminative detection of gaseous phosgene and a nerve agent mimic. Anal Chem 91:12070–12076
Dale TJ, Rebek J (2009) Hydroxy oximes as organophosphorus nerve agent sensors. Angew Chem Int Ed 48:7850–7852
Wu X, Wu Z, Han S (2011) Chromogenic and fluorogenic detection of a nerve agent simulant with a rhodamine-deoxylactam based sensor. Chem Commun 47:11468–11470
Kim TI, Maity SB, Bouffard J, Kim Y (2016) Molecular rotors for the detection of chemical warfare agent simulants. Anal Chem 88:9259–9263
Lu Z, Fan W, Shi X, Black CA, Fan C, Wang F (2018) A highly specific BODIPY-based fluorescent probe for the detection of nerve-agent simulants. Sens Actuators B Chem 255:176–182
Sarkar HS, Ghosh A, Das S, Maiti PK, Maitra S, Mandal S, Sahoo P (2018) Visualisation of DCP, a nerve agent mimic, in Catfish brain by a simple chemosensor. Sci Rep 8:3402
Mahapatra AK, Maiti K, Manna SK, Maji R, Mondal S, Mukhopadhyay CD, Sahoo P, Mandal D (2015) A cyclization-induced emission enhancement (CIEE)-based ratiometric fluorogenic and chromogenic probe for the facile detection of a nerve agent simulant DCP. Chem Commun 51:9729–9732
Mandal M, Guria UN, Halder S, Karak A, Banik D, Jana K, Kar A, Mahapatra AK (2022) A dual-channel chemodosimetric sensor for discrimination between hypochlorite and nerve-agent mimic DCP: application on human breast cancer cells. Org Biomol Chem 20:4803–4814
Prabhu K, Malode SJ, Kulkarni RM, Shetti NP (2022) Electro-sensing base for hazardous pesticide 2, 4-DCP and its quantification in real samples at ZnO@Cu core-shell nanoparticles in the presence of cationic surfactant. Mater Chem Phys 278:125705
Velmurugan K, Mathankumar S, Santoshkumar S, Amudha S, Nandhakumar R (2015) Specific fluorescent sensing of aluminium using naphthalene benzimidazole derivative in aqueous media. Spectrochim Acta A 139:119–123
Cao W, Zheng XJ, Fang DC, Jin LP (2015) Metal ion-assisted ring-opening of a quinazoline-based chemosensor: detection of copper(II) in aqueous media. Dalton Trans 44:5191–5196
Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Kim JS, Quang DT (2007) Calixarene-derived fluorescent probes. Chem Rev 107:3780–3799
Angell SE, Rogers CW, Zhang Y, Wolf MO, Jones WE (2006) Hemilabile coordination complexes for sensing applications. Coord Chem Rev 250:1829–1841
Rogers CW, Wolf MO (2002) Luminescent molecular sensors based on analyte coordination to transition-metal complexes. Coord Chem Rev 233–234:341–350
Wu D, Sedgwick AC, Gunnlaugsson T, Akkaya EU, Yoon J, James TD (2017) Fluorescent chemosensors: the past, present and future. Chem Soc Rev 46:7105–7123
Thomas SW III, Joly GD, Swager TM (2007) Chemical sensors based on amplifying fluorescent conjugated polymers. Chem Rev 107:339–1386
Xu W, Fu Y, Yao J, Fan T, Gao Y, He Q, Zhu D, Cao H, Cheng J (2016) Aggregation state reactivity activation of intramolecular charge transfer type fluorescent probe and application in trace vapor detection of sarin mimics. ACS Sens 1:1054–1059
Cai YC, Li C, Song QH (2017) Fluorescent chemosensors with varying degrees of intramolecular charge transfer for detection of a nerve agent mimic in solutions and in vapor. ACS Sens 2:834–841
Quang DT, Kim JS (2010) Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem Rev 110:6280–6301
Kaur B, Kaur N, Kumar S (2018) Colorimetric metal ions sensors- a comprehensive review of the years 2011–2016. Coord Chem Rev 358:13–69
Kaur K, Saini R, Kumar A, Luxami V, Kaur N, Singh P, Kumar S (2012) Chemodosimeters: an approach for detection and estimation of biologically and medically relevant metal ions, anions and thiols. Coord Chem Rev 256:1992–2028
Carter KP, Young AM, Palmer AE (2014) Fluorescent sensors for measuring metal ions in living systems. Chem Rev 114:4564–4601
Devaraj S, Saravanakumar D, Kandaswamy M (2009) Dual responsive chemosensors for anion and cation: synthesis and studies of selective chemosensor for F- and Cu(II) ions. Sens Actuators B 136:13–19
Fitzgerald WF, Lamgorg CH, Hammerschmidt CR (2007) Marine biogeochemical cycling of mercury. Chem Rev 107:641–662
Rani BK, John SA (2018) Fluorogenic mercury ion sensor based on pyrene-amino mercapto thiadiazole unit. J Hazard Mater 343:98–106
Gaggelli E, Kozlowski H, Valensin D, Valensin G (2006) Copper homeostasis neurodegenrative disorders (Alzheimer’s, Prion, and Parkinson’s Diseases and Amyotrophic Lateral Sclerosis). Chem Rev 106:1995–2044
Crabb E, Moore LE (2010) Smart Concepts in Transition Metal Chemistry, 1st edn. RSC Publishing, Cambridge
Harris ED (2001) Copper and Iron: a landmark connection of two essential metals. J Trace Elem Exp Med 14:207–210
Andreini C, Banci L, Bertini I, Rosato A (2008) Occurrence of copper proteins through the three domains of life: a bioinformatic approach. J Proteome Res 7:209–216
Que EL, Domaille DW, Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 108:1517–1549
Xu Z, Baek KH, Kim H, Cui J, Qian X, Spring DR, Shin I, Yoon J (2010) Zn2+-triggered amide tautomerization produces a Highly Zn2+-selective, cell-permeable, and ratiometric fluorescent sensor. J Am Chem Soc 132:601–610
Strausak D, Mercer JFB, Dieter HH, Stremmel W, Multhaup G (2001) Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res Bull 55:175–185
Muthaup G, Schlicksupp A, Hess L, Beher D, Ruppert T, Masters CL, Beyreuther K (1996) The amyloid precursor protein of Alzheimer’s disease in the reduction of copper(II) to copper(I). Science 271:1406–1409
Lovstad RA (2004) A kinetic study on the distribution of Cu (II)-ions between albumin and transferring. Biometals 17:111–113
Das R, Bej S, Ghosh D, Murmu NC, Hirani H, Banerjee P (2021) Stimuli-responsive discriminative detection of Cu2+ and Hg2+ with concurrent sensing of S2- from aqueous medium and bio-fluids by C-N fused azophenine functionalized “smart” hydrogel assay @A potential biomarker sensor for Wilson’s disease. Sens Actuators B Chem 341:129925
Kaur N (2014) Supramolecular switches-advanced molecular logic and computation molecular logic gates. Curr Org Chem 18:2892–2909
Kaur N, Singh J, Dhaka G, Rani R, Luxami V (2015) Benzothiazole-based chemosensor for CN- and Cu2+: multi-logic operations within a single molecule. Supramol Chem 27:453–459
Kaur N, Dhaka G, Singh J (2015) Hg2+-induced deprotonation of an anthracene-based chemosensor: set-reset flip-flop at the molecular level using Hg2+ and I- ions. New J Chem 39:6125–6129
Kaur N, Jindal G, Sukhvinder KS (2019) Cascade recognition of Hg2+ and cysteine using a naphthalene based ESIPT sensor and its application in a set/reset memorized device. New J Chem 43:436–443
Kaur N, Kaur G, Alreja P (2018) 1, 10-Phenanthroline based ESIPT sensor for cascade recognition of Cu2+ and CN− ions. J Photochem Photobiol A Chem 353:138–142
Sahoo SK, Sharma D, Moirangthem A, Kuba A, Thomas R, Kumar R, Kuwar A, Choi HJ, Basu A (2016) Pyridoxal derived chemosensor for chromogenic sensing of Cu2+ and fluorogenic sensing of Fe3+ in semi-aqueous medium. J Luminesc 172:297–303
Acha ND, Elosua C, Corres JM, Arregui FJ (2019) Fluorescent sensors for the detection of heavy metal ions in aqueous media. Sensors 19:599–633
Hu JH, Li JB, Qi J, Chen JJ (2015) Highly selective and effective mercury(II) fluorescent sensors. New J Chem 39:843–848
Deka S, Guha AK, Das DK (2021) Fluorescent sensors for Hg2+ and Cu2+ based on condensation products of 4H 1,2,4 Triazole 4 amine and carboxylated benzoic acids. J Fluoresc 31:1937–1945
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Long GL (1983) Limit of detection. a closer look at the IUPAC definition. Anal Chem 55:712A-724A
Goswami S, Chakrabarty R (2011) An imidazole based colorimetric sensor for fluoride anion. Eur J Chem 2(3):410–415
Jindal G, Kaur N (2022) Fluorimetric quantification of picric acid in aqueous medium via smartphone and invisible ink applications using pyrene based sensor. Inorg Chem Comm 140:109481
Razi SS, Gupta RC, Ali R, Dwivedi SK, Srivastava P, Misra A (2016) A new D-π-A type intramolecular charge transfer Dyad System to detect F−: Anion induced CO2 sensing. Sens Actuators B 236:520–528
Ali SS, Gangopadhyay A, Pramanik AK, Guria UN, Samanta SK, Mahapatra AK (2019) Ratiometric sensing of nerve agent mimic DCP through in situ benzisoxazole formation. Dyes Pigm 170:107585
Qin T, Huang Y, Zhu K, Wang J, Pan C, Liu B, Wang L (2019) A flavonoid-based fluorescent test strip for sensitive and selective detection of a gaseous nerve agent simulant. Anal Chim Acta 1076:125–130
Dagnaw FW, Feng W, Song QH (2020) Selective and rapid detection of nerve agent simulants by polymer fibers with a fluorescent chemosensor in gas phase. Sens Actuators B Chem 318:127937
Hu X, Zeng H, Chen T, Yuan HQ, Zeng L, Bao GM (2020) Fast and visual detection of a chemical warfare agent mimic using a simple, effective and portable chemodosimeter. Sens Actuators B Chem 319:128282
Ganesan JS, Gandhi S, Radhakrishnan K, Balasubramaniem A, Sepperumal M, Ayyanar S (2019) Execution of julolidine based derivative as bifunctional chemosensor for Zn2+ and Cu2+ ions: applications in bio-imaging and molecular logic gate. Spectrochim Acta A 219:33–43
Uchacz T, Szlachcic P, Danel A, Kukułka M, Srebro-Hooper M, Stopa G, Stadnicka KM (2019) Photophysical properties of 1-pyridine-3-phenylpyrazoloquinoline and molecular logic gate implementation. Dyes Pigm 166:490–501
Li WT, Wu GY, Qu WJ, Li Q, Lou JC, Lin Q, Yao H, Zhang YM, Wei TB (2017) A colorimetric and reversible fluorescent chemosensor for Ag+ in aqueous solution and its application in IMPLICATION logic gate. Sens Actuators B Chem 239:671–678
Liu L, Liu P, Ga L, Ai J (2021) Advances in applications of molecular logic gates. ACS Omega 6:30189–30204
Ceroni P, Bergamini G, Balzani V (2009) Old molecules, new concepts: [Ru(bpy)3]2+ as a molecular encoder decoder. Angew Chem Int Ed 48:8516–8518
Acknowledgements
For recording the NMR, FTIR and HRMS spectra, the authors are gratified to SAIF, Panjab University Chandigarh.
Funding
The authors are greatly obliged to CSIR-New Delhi (File No.-09/135(0911)2020 EMR-I) and DST PURSE-II (Grant—48/RPC) for funding.
Author information
Authors and Affiliations
Contributions
Gitanjali Jindal contributed towards conceptualization, data curation, formal analysis, visualization, writing-original draft. Navneet Kaur gave contribution for methodology, writing-review & editing, supervision, project administration.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
The authors declare that they consent to participate.
Consent for Publication
The authors provide consent for the publication.
Conflicts of Interest
The authors have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jindal, G., Kaur, N. Fluorimetric Recognition of Nerve Agent Mimic Diethylchlorophosphate Along with Cu2+/Hg2+ Ions Using Imidazole Possessing Sensor. J Fluoresc 33, 359–371 (2023). https://doi.org/10.1007/s10895-022-03069-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03069-z