Abstract
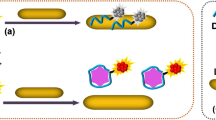
Upconversion nanoparticles (UCNPs) have achieved considerable success in protein sensing in vitro. And aptamer is one of the most frequently used biomolecules to modify the nanoparticles for protein assay. However, the complicated process of modifying UCNPs with DNA and the susceptibility of the phosphate groups of DNA backbone to adsorb on the surface of UCNPs have limited their practical applications. To overcome these limitations, a modification-free fluorescent biosensor based on polydopamine-coated upconversion nanoparticles (UCNPs@PDA) is proposed. It consists of UCNPs@PDA and CEA aptamer-functionalized AuNPs (AuNPs-CEA aptamer). The CEA aptamer on AuNPs can be adsorbed onto the surface of UCNPs@PDA due to the interactions of π–π stacking and hydrogen bonding, triggering the process of fluorescence resonance energy transfer (FRET) from UCNPs@PDA to AuNPs-CEA aptamer. In the presence of CEA, the AuNPs-CEA aptamer departs from UCNPs@PDA due to the stronger affinity of CEA with its aptamer. Therefore, the recovery of upconversion fluorescence can sensitively quantify the concentration of CEA. This biosensor provides a linear range from 0.1 to 100 ng/mL for CEA with a LOD of 0.031 ng/mL in an aqueous solution. In spiked human serum samples, the same linear range is acquired with a slightly higher LOD of 0.055 ng/mL, demonstrating the great potential of the biosensor in practical application.







Similar content being viewed by others
Data Availability
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.
References
Zheng W, Huang P, Tu DT, Ma E, Zhu HM, Chen XY (2015) Lanthanide-doped upconversion nano-bioprobes: electronic structures, optical properties, and biodetection. Chem Soc Rev 44:1379–1415
Auzel F (2004) Upconversion and anti-Stokes processes with f and d ions in solids. Chem Rev 104:139–173
Su QQ, Feng W, Yang DP, Li FY (2017) Resonance energy transfer in upconversion nanoplatforms for selective biodetection. Acc Chem Res 50:32–40
Zhang YQ, Xu S, Li XP, Zhang JS, Sun J, Tong LL, Zhong H, Xia HP, Hua RN, Chen BJ (2018) Improved LRET-based detection characters of Cu2+ using sandwich structured NaYF4@NaYF4:Er3+/Yb3+@NaYF4 nanoparticles as energy donor. Sensors and Actuators B-Chemical 257:829–838
Doughan S, Uddayasankar U, Krull UJ (2015) A paper-based resonance energy transfer nucleic acid hybridization assay using upconversion nanoparticles as donors and quantum dots as acceptors. Anal Chim Acta 878:1–8
Wu ZJ, Li H, Liu ZH (2015) An aptasensor for carcinoembryonic antigen based on upconversion fluorescence resonance energy transfer. Sensors and Actuators B-Chemical 206:531–537
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science (New York, N.Y.) 249:505–510
Stoltenburg R, Reinemann C, Strehlitz B (2007) SELEX–a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng 24:381–403
Fang XH, Tan WH (2010) Aptamers generated from Cell-SELEX for molecular medicine: A chemical biology approach. Acc Chem Res 43:48–57
Mairal T, Ozalp VC, Sanchez PL, Mir M, Katakis I, O’Sullivan CK (2008) Aptamers: molecular tools for analytical applications. Anal Bioanal Chem 390:989–1007
Keefe AD, Pai S, Ellington A (2010) Aptamers as therapeutics (vol 9, pg 537, 2010). Nat Rev Drug Discovery 9:8
Wang RW, Zhu GZ, Mei L, Xie Y, Ma HB, Ye M, Qing FL, Tan WH (2014) Automated modular synthesis of aptamer-drug conjugates for targeted drug delivery. J Am Chem Soc 136:2731–2734
Hicke BJ, Stephens AW, Gould T, Chang Y-F, Lynott CK, Heil J, Borkowski S, Hilger C-S, Cook G, Warren S, Schmidt PG (2006) Tumor targeting by an aptamer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 47:668–678
Li S, Xu LG, Sun MZ, Wu XL, Liu LQ, Kuang H, Xu CL (2017) Hybrid nanoparticle pyramids for intracellular dual microRNAs biosensing and bioimaging. Adv Mater 29:19
Yu Z, Ge Y, Sun Q, Pan W, Wan X, Li N, Tang B (2018) A pre-protective strategy for precise tumor targeting and efficient photodynamic therapy with a switchable DNA/upconversion nanocomposite. Chem Sci 9:3563–3569
Chen GY, Qju HL, Prasad PN, Chen XY (2014) Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem Rev 114:5161–5214
Perlin L, MacNeil S, Rimmer S (2008) Production and performance of biomaterials containing RGD peptides. Soft Matter 4:2331–2349
Han S, Samanta A, Xie XJ, Huang L, Peng JJ, Park SJ, Teh DBL, Choi Y, Chang YT, All AH, Yang YM, Xing BG, Liu XG (2017) Gold and hairpin DNA functionalization of upconversion nanocrystals for imaging and in vivo drug delivery. Adv Mater 29:18
Qiang WB, Li W, Li XQ, Chen X, Xu DK (2014) Bioinspired polydopamine nanospheres: a superquencher for fluorescence sensing of biomolecules. Chem Sci 5:3018–3024
Liu XS, Cao JM, Li H, Li JY, Jin Q, Ren KF, Ji J (2013) Mussel-inspired polydopamine: a biocompatible and ultrastable coating for nanoparticles in vivo. ACS Nano 7:9384–9395
Ma SS, Qi YX, Jiang XQ, Chen JQ, Zhou QY, Shi GY, Zhang M (2016) Selective and sensitive monitoring of cerebral antioxidants based on the dye-labeled DNA/polydopamine conjugates. Anal Chem 88:11647–11653
Hammarstrom S, Engvall E, Johansson BG, Svensson S, Sundblad G, Goldstein IJ (1975) Nature of the tumor-associated determinant(s) of carcinoembryonic antigen. Proc Natl Acad Sci USA 72:1528–1532
Saha K, Agasti SS, Kim C, Li XN, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112:2739–2779
Zhang KY, Yang L, Lu F, Wu XC, Zhu JJ (2018) A universal upconversion sensing platform for the sensitive detection of tumour-related ncRNA through an exo III-assisted cycling amplification strategy. Small 14:10
Wang F, Deng RR, Liu XG (2014) Preparation of core-shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat Protoc 9:1634–1644
Liu FY, He XX, Lei Z, Liu L, Zhang JP, You HP, Zhang HM, Wang ZX (2015) Facile preparation of doxorubicin-loaded upconversion@polydopamine nanoplatforms for simultaneous in vivo multimodality imaging and chemophotothermal synergistic therapy. Adv Healthcare Mater 4:559–568
Haiss W, Thanh NTK, Aveyard J, Fernig DG (2007) Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal Chem 79:4215–4221
Liu J, Lu Y (2006) Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat Protoc 1:246–252
Zheng M, Jagota A, Semke ED, Diner BA, McLean RS, Lustig SR, Richardson RE, Tassi NG (2003) DNA-assisted dispersion and separation of carbon nanotubes. Nat Mater 2:338–342
Wang CL, Li XM, Zhang F (2016) Bioapplications and biotechnologies of upconversion nanoparticle-based nanosensors. Analyst 141:3601–3620
Wang DF, Li YY, Lin ZY, Qiu B, Guo LH (2015) Surface-enhanced electrochemiluminescence of Ru@SiO2 for ultrasensitive detection of carcinoembryonic antigen. Anal Chem 87:5966–5972
Funding
This work was supported by [National Natural Science Foundation of China] [21906124] and Graduate Innovative Fund of Wuhan Institute of Technology.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design.Material preparation, data collection and analysis were performed by Yu,Tang and Qiu. The first draft of the manuscript was edited by Zha and Liu. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Statement
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of interest
The authors declare they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, D., Zha, Z., Tang, S. et al. Modification-Free Fluorescent Biosensor for CEA Based on Polydopamine-Coated Upconversion Nanoparticles. J Fluoresc 32, 1289–1297 (2022). https://doi.org/10.1007/s10895-022-02973-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-02973-8