Abstract
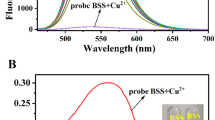
Here, three Schiff bases 3a-c, differing by the substitutions (–H, –Cl, and –N(CH3)2) on the phenyl ring, have been designed and synthesized via the reaction of ortho-aminophenol with benzaldehyde, 2,4-dichlorobenzaldehyde and para-dimethylamine benzaldehyde in 1:1 molar ratio with favourable yields of 89–92%, respectively. Their structural characterizations were studied by FT-IR, NMR, MALDI-MS and elemental analysis. The fluorescence behaviours of compounds 3a and 3b exhibited a severe aggregation caused quenching (ACQ) effect in EtOH/water system. On the contrary, compound 3c had an obvious J-aggregation induced emission (AIE) feature in EtOH/water mixture (v/v = 1:1), and exhibited excellent sensitivity and anti-interference towards Cu2+ with the limit of detection (LOD) of 1.35 × 10–8 M. Job’s plot analysis and MS spectroscopic study revealed the 2:1 complexation of probe 3c and Cu2+. In addition, probe 3c was successfully applied to the determination of Cu2+ in real aqueous samples.









Similar content being viewed by others
Data Availability Statement
Data and material information is provided and will be shared on request.
References
Wang L, Si W, Ye Y, Wang S, Hou F, Hou X, Cai H, Dou SX, Liang J (2021) Cu-ion-implanted and polymeric carbon nitride-decorated TiO2 nanotube array for unassisted photoelectrochemical water splitting. ACS Appl Mater Interfaces 13:44184–44194. https://doi.org/10.1021/acsami.1c09665
Liu Y, Nie N, Tang H, Zhang C, Chen K, Wang W, Liu J (2021) Effective antibacterial activity of degradable copper-doped phosphate-based glass nanozymes. ACS Appl Mater Interfaces 13:11631–11645. https://doi.org/10.1021/acsami.0c22746
Zhu Q, Cheng M, Zhang B, Jin K, Chen S, Ren Z, Yu Y (2019) Realizing a rechargeable high-performance Cu–Zn battery by adjusting the solubility of Cu2+. Adv Funct Mater 29:1905979. https://doi.org/10.1002/adfm.201905979
Bolisetty S, Peydayesh M, Mezzenga R (2019) Sustainable technologies for water purification from heavy metals: review and analysis. Chem Soc Rev 48:463–487. https://doi.org/10.1039/C8CS00493E
Wang S, Sheng Z, Yang Z, Hu D, Long X, Feng G, Liu Y, Yuan Z, Zhang J, Zheng H, Zhang X (2019) Activatable small-molecule photoacoustic probes that cross the blood–brain barrier for visualization of copper(II) in mice with Alzheimer’s disease. Angew Chem Int Ed 58:12415–12419. https://doi.org/10.1002/anie.201904047
Liu Y, Su Q, Chen M, Dong Y, Shi Y, Feng W, Wu ZY, Li F (2016) Near-infrared upconversion chemodosimeter for in vivo detection of Cu2+ in Wilson disease. Adv Mater 28:6625–6630. https://doi.org/10.1002/adma.201601140
Lee S, Chung CY, Liu P, Craciun L, Nishikawa Y, Bruemmer KJ, Hamachi I, Saijo K, Miller EW, Chang CJ (2020) Activity-based sensing with a metal-directed acyl imidazole strategy reveals cell type-dependent pools of labile brain copper. J Am Chem Soc 142:14993–15003. https://doi.org/10.1021/jacs.0c05727
Udhayakumari D, Naha S, Velmathi S (2017) Colorimetric and fluorescent chemosensors for Cu2+. A comprehensive review from the years 2013–15. Anal Methods 9:552–578. https://doi.org/10.1039/C6AY02416E
Saleem M, Lee KH (2015) Optical sensor: a promising strategy for environmental and biomedical monitoring of ionic species. RSC Adv 5:72150–72287. https://doi.org/10.1039/C5RA11388A
Kowser Z, Rayhan U, Akther T, Redshaw C, Yamato T (2021) A brief review on novel pyrene based fluorometric and colorimetric chemosensors for the detection of Cu2+. Mater Chem Front 5:2173–2200. https://doi.org/10.1039/D0QM01008A
Sharma P, Kaur S, Kaur S, Singh P (2020) Near-IR oxime-based solvatochromic perylene diimide probe as a chemosensor for Pd species and Cu2+ ions in water and live cells. Photochem Photobiol Sci 19:504–514. https://doi.org/10.1039/C9PP00487D
Singh P, Mittal LS, Kumar S, Bhargava G, Kumar S (2014) Perylene diimide appended with 8-hydroxyquinoline for ratiometric detection of Cu2+ ions and metal displacement driven “turn on” cyanide sensing. J Fluoresc 24:909–915. https://doi.org/10.1007/s10895-014-1371-6
Xu T, Huang J, Fang M, Sui M, Zhu Y, Shentu Y, Li C, Zhu W (2020) A novel “turn-on” fluorescent probe based on naphthalimide for the tracking of lysosomal Cu2+ in living cells. New J Chem 44:21167–21175. https://doi.org/10.1039/D0NJ04416D
Wei J, Sun H, Jiang Y, Miao B, Han X, Zhao Y, Ni Z (2021) A novel 1,8-naphthalimide-based Cu2+ ion fluorescent probe and its bioimaging application. Spectrochim Acta A Mol Biomol Spectrosc 261:120037. https://doi.org/10.1016/j.saa.2021.120037
Wang X, Shao J, Hu Y (2019) An efficient chemodosimeter for Cu(II) ions based on hydrolysis of fluorescein and its utility in live cell imaging. Dyes Pigm 171:107680. https://doi.org/10.1016/j.dyepig.2019.107680
He G, Zhao X, Zhang X, Fan H, Wu S, Li H, He C, Duan C (2010) A turn-on PET fluorescence sensor for imaging Cu2+ in living cells. New J Chem 34:1055–1058. https://doi.org/10.1039/C0NJ00132E
Warrier SB, Kharkar PS (2018) A coumarin based chemosensor for selective determination of Cu (II) ions based on fluorescence quenching. J Lumin 199:407–415. https://doi.org/10.1016/j.jlumin.2018.03.073
Jung HS, Kwon PS, Lee JW, Kim JI, Hong CS, Kim JW, Yan S, Lee JY, Lee JH, Joo T, Kim JS (2009) Coumarin-derived Cu2+-selective fluorescence sensor: Synthesis, mechanisms, and applications in living cells. J Am Chem Soc 131:2008–2012. https://doi.org/10.1021/ja808611d
Chawla HM, Munjal P, Goel P (2015) Synthesis and evaluation of a new colorimetric and ratiometric fluorescence probe for copper ions. J Lumin 164:138–145. https://doi.org/10.1016/j.jlumin.2015.03.027
Ramachandran M, Anandan S, Ashokkumar M (2019) A luminescent on–off probe based calix[4]arene linked through triazole with ruthenium (II) polypyridine complexes to sense copper (II) and sulfide ions. New J Chem 43:9832–9842. https://doi.org/10.1039/C9NJ01632E
Chan WC, Saad HM, Sim KS, Lee VS, Ang CW, Yeong KY, Tan KW (2021) A rhodamine based chemosensor for solvent dependent chromogenic sensing of cobalt (II) and copper (II) ions with good selectivity and sensitivity: Synthesis, filter paper test strip, DFT calculations and cytotoxicity. Spectrochim Acta A Mol Biomol Spectrosc 262:120099. https://doi.org/10.1016/j.saa.2021.120099
Mishra SK, Dehuri S, Bag B (2020) Effect of n-alkyl substitution on Cu(II)-selective chemosensing of rhodamine B derivatives. Org Biomol Chem 18:316–332. https://doi.org/10.1039/C9OB02439E
Shen Y, Zheng W, Yao Y, Wang D, Lv G, Li C (2020) Phenoxazine-based near-infrared fluorescent probes for the specific detection of copper (II) ions in living cells. Chem Asian J 15:2864–2867. https://doi.org/10.1002/asia.202000783
Ravichandiran P, Prabakaran DS, Maroli N, Boguszewska-Czubara A, Masłyk M, Kim AR, Chandrasekaran B, Yoo DJ (2021) Construction of a simple dual-channel fluorescence chemosensor for Cu2+ ion and GSSG detection and its mitochondria-targeting bioimaging applications. Anal Chim Acta 1181:338896. https://doi.org/10.1016/j.aca.2021.338896
Baslak C, Kursunlu AN (2018) A naked-eye fluorescent sensor for copper (II) ions based on a naphthalene conjugate Bodipy dye. Photochem Photobiol Sci 17:1091–1097. https://doi.org/10.1039/C8PP00137E
Xie HR, Gu YQ, Liu L, Dai JC (2020) A H-aggregating fluorescent probe for recognizing both mercury and copper ions based on a dicarboxyl-pyridyl bifunctionalized difluoroboron dipyrromethene. New J Chem 44:19713–19722. https://doi.org/10.1039/D0NJ04124F
Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ (2006) A selective turn-on fluorescent sensor for imaging copper in living cells. J Am Chem Soc 128:10–11. https://doi.org/10.1021/ja055064u
Chen Y, Pan H, Wang F, Zhao Y, Yin H, Chen Y, Zhang J, Jiang J (2019) An ultrafast BODIPY single molecular sensor for multi-analytes (acid/base/Cu2+/Bi3+) with different sensing mechanism. Dyes Pigm 165:279–286. https://doi.org/10.1016/j.dyepig.2019.02.034
Acharyya K, Mukherjee PS (2014) A fluorescent organic cage for picric acid detection. Chem Commun 50:15788–15791. https://doi.org/10.1039/C4CC06225F
Li A, Liu Y, Zhou W, Jiang Y, He Q (2022) Superphanes: Facile and efficient preparation, functionalization and unique properties. Tetrahedron Chem 1:100006. https://doi.org/10.1016/j.tchem.2022.100006
Zhu M, Guo H, Cai K, Yang F, Wang Z (2017) Novel fluorescence-enhancing effect based on light-induced E/Z isomerization of perylene diimides with Schiff-base groups on bay-positions. Dyes Pigm 140:179–186. https://doi.org/10.1016/j.dyepig.2017.01.048
Abdel-Kader NS, Moustafa H, El-Ansary AL, Sherif OE, Farghaly AM (2021) A coumarin Schiff base and its AG (I) and CU (II) complexes: synthesis, characterization, DFT calculations and biological applications. New J Chem 45:7714–7730. https://doi.org/10.1039/D0NJ05688J
Cantón-Díaz AM, Muñoz-Flores BM, Moggio I, Arias E, León AD, García-López MC, Santillán R, Ochoa ME, Jiménez-Pérez VM (2018) One-pot microwave-assisted synthesis of organotin Schiff bases: an optical and electrochemical study towards their effects in organic solar cells. New J Chem 42:14586–14596. https://doi.org/10.1039/C8NJ02998A
Petrus ML, Music A, Closs AC, Bijleveld JC, Sirtl MT, Hu Y, Dingemans TJ, Bein T, Docampo P (2017) Design rules for the preparation of low-cost hole transporting materials for perovskite solar cells with moisture barrier properties. J Mater Chem A 5:25200–25210. https://doi.org/10.1039/C7TA06452G
Panda U, Roy S, Mallick D, Layek A, Ray PP, Sinha C (2017) Aggregation induced emission enhancement (AIEE) of fluorenyl appended Schiff base : A turn on fluorescent probe for Al3+, and its photovoltaic effect. J Lumin 181:56–62. https://doi.org/10.1016/j.jlumin.2016.07.008
Zhu M, Zhong M, Chen M, Huang S, Li Y, Cao F (2022) A π-conjugated α-cyanostilbene dimer emitting strongly red fluorescence with a large Stokes’ shift of ca. 300 nm and used as a probe for selective detection of Cu2+. Opt Mater 125:112059. https://doi.org/10.1016/j.optmat.2022.112059
Goshisht MK, Patra GK, Tripathi N (2022) Fluorescent Schiff base sensors as a versatile tool for metal ion detection: strategies, mechanistic insights, and applications. Mater Adv. https://doi.org/10.1039/D1MA01175H
Huang S, Zheng L, Zheng S, Guo H, Yang F (2022) First fluorescence sensor for hydrazine ion: An effective “turn-on” detection based on thiophene-cyanodistyrene Schiff-base. J Photochem Photobiol A 427:113851. https://doi.org/10.1016/j.jphotochem.2022.113851
Zha B, Fang S, Chen H, Guo H, Yang F (2022) An effective dual sensor for Cu2+ and Zn2+ with long-wavelength fluorescence in aqueous media based on biphenylacrylonitrile Schiff-base. Spectrochim Acta A Mol Biomol Spectrosc 269:120765. https://doi.org/10.1016/j.saa.2021.120765
Yuan T, Fei J, Xu Y, Yang X, Li J (2017) Stimuli-responsive dipeptide–protein hydrogels through Schiff base coassembly. Macromol Rapid Commun 38:1700408. https://doi.org/10.1002/marc.201700408
Jia Y, Li J (2015) Molecular assembly of Schiff base interactions: construction and application. Chem Rev 115:1597–1621. https://doi.org/10.1021/cr400559g
Jiang S, Guo H, Zhu S, Yang F (2017) Novel columnar liquid crystalline oligomers: Triphenylene tetramers with rigid aromatic Schiff-bases and hydrogen-bonding spacers via click chemistry. J Mol Liq 236:76–80. https://doi.org/10.1016/j.molliq.2017.04.007
Han C, Guo H, Lai J, Yang F (2017) Calix[4]resorcinarene-cholesterol columnar liquid crystals: Synthesis, mesomorphism and the influence of spacers on liquid crystalline behaviors. J Mol Liq 231:220–224. https://doi.org/10.1016/j.molliq.2017.01.111
Kundu BK, Mandal P, Mukhopadhyay BG, Tiwari R, Nayak D, Ganguly R, Mukhopadhyay S (2019) Substituent dependent sensing behavior of Schiff base chemosensors in detecting Zn2+ and Al3+ ions: Drug sample analysis and living cell imaging. Sens Actuators B-Chem 282:347–358. https://doi.org/10.1016/j.snb.2018.11.076
Huang Z, Zhang X, Zhang X, Yang B, Zhang Y, Wang K, Yuan J, Tao L, Wei Y (2015) One-pot synthesis and biological imaging application of an amphiphilic fluorescent copolymer via a combination of RAFT polymerization and Schiff base reaction. Polym Chem 6:2133–2138. https://doi.org/10.1039/C4PY01769B
Cheng J, Li Y, Sun R, Liu J, Gou F, Zhou X, Xiang H, Liu J (2015) Functionalized Salen ligands linking with non-conjugated bridges: unique and colorful aggregation-induced emission, mechanism, and applications. J Mater Chem C 3:11099–11110. https://doi.org/10.1039/C5TC02555A
Cheng J, Zhang Y, Ma X, Zhou X, Xiang H (2013) Colorimetric and fluorescent pH and Cu2+ probes induced by photoisomerization of a maleonitrile-based Salen ligand. Chem Commun 49:11791–11793. https://doi.org/10.1039/C3CC47137C
Zheng H, Kang Y, Wu M, Liang Q, Zheng J, Zheng X, Jin L (2021) ESIPT-AIE active Schiff base based on 2-(2′-hydroxyphenyl)benzo-thiazole applied as multi-functional fluorescent chemosensors. Dalton Trans 50:3916–3922. https://doi.org/10.1039/D1DT00241D
Padhan SK, Murmu N, Mahapatra S, Dalai MK, Sahu SN (2019) Ultrasensitive detection of aqueous Cu2+ ions by a coumarin-salicylidene based AIEgen. Mater Chem Front 3:2437–2447. https://doi.org/10.1039/C9QM00394K
Barot YB, Anand V, Mishra R (2022) Di-Triphenylamine-based AIE active Schiff base for highly sensitive and selective fluorescence sensing of Cu2+ and Fe3+. J Photochem Photobiol A 426:113785. https://doi.org/10.1016/j.jphotochem.2022.113785
Li N, Xue J, Zhang X, Shi N, Liu W, Wu R, Fan C, Xu C, Bi S, Fan Y (2021) A novel dimer-induced AIE material as a nano-sensor for colormetric and ratiometric sensing of Erythromycin and metal ions (Zn2+, Cd2+ and Cu2+) with different dissociation and re-aggregation processes and cellular imaging applications. Dyes Pigm 184:108872. https://doi.org/10.1016/j.dyepig.2020.108872
Xu Z, Wang Y, Wang Y, Li J, Luo W, Wu W, Fan Y (2019) AIE active salicylaldehyde-based hydrazone: A novel single-molecule multianalyte (Al3+ or Cu2+) sensor in different solvents. Spectrochim Acta A Mol Biomol Spectrosc 212:146–154. https://doi.org/10.1016/j.saa.2019.01.003
Zhu M, Chen Y, Zhang X, Chen M, Guo H, Yang F (2018) Perylene bisimide with diphenylacrylonitrile on side-chain: strongly fluorescent liquid crystal with large pseudo Stokes shift based on AIE and FRET effect. Soft Matter 14:6737–6744. https://doi.org/10.1039/C8SM01183D
Zhang Y, Yuan S, Liu P, Jing L, Pan H, Ren X, Chen Z (2021) J-aggregation induced emission enhancement of BODIPY dyes via H-bonding directed supramolecular polymerization: the importance of substituents at boron. Org Chem Front 8:4078–4085. https://doi.org/10.1039/D1QO00520K
Lu H, Xue Z, Mack J, Shen Z, You X, Kobayashi N (2010) Specific Cu2+-induced J-aggregation and Hg2+-induced fluorescence enhancement based on BODIPY. Chem Commun 46:3565–3567. https://doi.org/10.1039/B926300D
Acknowledgements
We are grateful for the financial support from the Undergraduate Innovation Program in Neijiang Normal University (No. X2021022 and No. X2021179), the Scientific Research Program in Neijiang Normal University (No. X20B0015).
Funding
The study was supported by the Undergraduate Innovation Program in Neijiang Normal University (No. X2021022 and No. X2021179), the Scientific Research Program in Neijiang Normal University (No. X20B0015).
Author information
Authors and Affiliations
Contributions
MC: investigation and formal analysis of optical properties for target compounds; FC: syntheses of the target compounds; SH: investigation of sensing Cu2+; YL: investigation of recovery results of Cu2+ in real water samples; MZ: measurements of spectral properties; MZ: study design and writing-original draft.
Corresponding author
Ethics declarations
Ethics Approval
There is no ethic approval required for this research work.
Consent to Participate
Not applicable.
Consent for Publication
All authors agree for the publication.
Conflicts of Interest
No potential conflict of interest is reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, M., Cao, F., Huang, S. et al. The Schiff Base Probe With J-aggregation Induced Emission for Selective Detection of Cu2+. J Fluoresc 32, 1457–1469 (2022). https://doi.org/10.1007/s10895-022-02948-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-02948-9