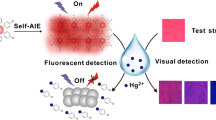
Abstract
A Fluorescent chemosensor based on pyrene scaffold, 5-diethylamino-2-(pyren-1-yliminomethyl)-phenol (PDS) is synthesized using condensation method. It displays novel aggregation-induced emission (AIE) phenomena in its aggregated/solid state. The AIE characteristic of PDS is studied in CH3CN/H2O mixtures at different volume percentage of water and morphology of the aggregated particles are investigated by DLS and optical fluorescence microscopic study. The probe is aggregated into ordered one-dimensional (1-D) rod like microcrystals and exhibit high efficiency of solid-state emission with green colour. By taking advantage of its interesting AIE feature, the aggregated hydrosol has been utilized as ‘off–on’ type fluorescence switching chemosensor with superb selectivity and sensitivity towards Cu2+ions and the limit of detection (LOD) was calculated as low as 6.3 µM. A high Stern–Volmer quenching constant was estimated to be 2.88 × 105 M−1. The proposed chemosensor with AIE feature reveals a prospective view for the on-site visual recognition of Cu2+ ions in fluorescent paper strips and the synthesized probe is also exploited to find out the concentration of Cu2+ions in real water samples.














Similar content being viewed by others
Data Availability
Raw data and materials are available on request to the author.
References
Xiao J, Yang B, Wong JI, Liu Y, Wei F, Tan KJ, Zhang Q (2011) Synthesis, characterization, self-assembly, and physical properties of 11-methylbenzo [d] pyreno [4, 5-b] furan. Org Lett 13(12):3004–3007
Yuan WZ, Gong Y, Chen S, Shen XY, Lam JW, Lu P, Tang BZ (2012) Efficient solid emitters with aggregation-induced emission and intramolecular charge transfer characteristics: molecular design, synthesis, photophysical behaviors, and OLED application. Chem Mater 24(8):1518–1528
Shyamal M, Maity S, Mazumdar P, Sahoo GP, Maity R, Misra A (2017) Synthesis of an efficient Pyrene based AIE active functional material for selective sensing of 2, 4, 6-trinitrophenol. J Photochem Photobiol A 342:1–14
Reineke S, Lindner F, Schwartz G, Seidler N, Walzer K, Lüssem B, Leo K (2009) White organic light-emitting diodes with fluorescent tube efficiency. Nature 459(7244):234–238
Shyamal M, Das D, Giri PK, Maity S, Misra A (2019) Aggregation-induced emission–based highly selective ‘turn-off’fluorogenic chemosensor for robust quantification of explosive picric acid in aqueous and solid states. Mater Today Chem 14:100193
Shyamal M, Maity S, Maity A, Maity R, Roy S, Misra A (2018) Aggregation induced emission based “turn-off” fluorescent chemosensor for selective and swift sensing of mercury (II) ions in water. Sens Actuators B Chem 263:347–359
Shyamal M, Mazumdar P, Maity S, Samanta S, Sahoo GP, Misra A (2016) Highly selective turn-on fluorogenic chemosensor for robust quantification of Zn (II) based on aggregation induced emission enhancement feature. ACS Sensors 1(6):739–747
Ma H, Yang M, Zhang C, Ma Y, Qin Y, Lei Z, Yang Y (2017) Aggregation-induced emission (AIE)-active fluorescent probes with multiple binding sites toward ATP sensing and live cell imaging. J Mater Chem B 5(43):8525–8531
Shustova NB, Cozzolino AF, Reineke S, Baldo M, Dincă M (2013) Selective turn-on ammonia sensing enabled by high-temperature fluorescence in metal–organic frameworks with open metal sites. J Am Chem Soc 135(36):13326–13329
Kachwal V, Alam P, Yadav HR, Pasha SS, Choudhury AR, Laskar IR (2018) Simple ratiometric push–pull with an ‘aggregation induced enhanced emission’active pyrene derivative: a multifunctional and highly sensitive fluorescent sensor. New J Chem 42(2):1133–1140
La DD, Bhosale SV, Jones LA, Bhosale SV (2017) Tetraphenylethylene-based AIE-active probes for sensing applications. ACS Appl Mater Interfaces 10(15):12189–12216
Mazumdar P, Maity S, Shyamal M, Das D, Sahoo GP, Misra A (2016) Proton triggered emission and selective sensing of picric acid by the fluorescent aggregates of 6, 7-dimethyl-2, 3-bis-(2-pyridyl)-quinoxaline. Phys Chem Chem Phys 18(10):7055–7067
Maity A, Mazumdar P, Samanta S, Das D, Shyamal M, Sahoo GP, Misra A (2016) Morphology directing synthesis of 1-aminopyrene microstructures and its super quenching effect towards nitro aromatics. J Mol Liq 221:358–367
Liu D, Wang Z, Jiang X (2011) Gold nanoparticles for the colorimetric and fluorescent detection of ions and small organic molecules. Nanoscale 3(4):1421–1433
Rao JP, Geckeler KE (2011) Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci 36(7):887–913
Kazlauskas K, Kreiza G, Arbačiauskienė E, Bieliauskas A, Getautis V, Šačkus A, Juršėnas S (2014) Morphology and emission tuning in fluorescent nanoparticles based on phenylenediacetonitrile. J Phys Chem 118(43):25261–25271
Maity S, Shyamal M, Mazumdar P, Sahoo GP, Maity R, Salgado-Morán G, Misra A (2016) Solvatochromism and turn-off fluorescence sensing property of N, N′-bis (3-pentyl) perylene-3, 4, 9, 10-bis (dicarboximide) towards nitroaromatics and photophysical study of its microstructures. J Mol Liq 224:255–264
Luo J, Xie Z, Lam JW, Cheng L, Chen H, Qiu C, Tang BZ (2001) Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem Commun 18:1740–1741
Chan CY, Zhao Z, Lam JW, Liu J, Chen S, Lu P, Tang BZ (2012) Efficient light emitters in the solid state: synthesis, aggregation-induced emission, electroluminescence, and sensory properties of luminogens with benzene cores and multiple triarylvinyl peripherals. Adv Func Mater 22(2):378–389
Chen J, Law CC, Lam JW, Dong Y, Lo SM, Williams ID, Tang BZ (2003) Synthesis, light emission, nanoaggregation, and restricted intramolecular rotation of 1, 1-substituted 2, 3, 4, 5-tetraphenylsiloles. Chem Mater 15(7):1535–1546
An BK, Kwon SK, Jung SD, Park SY (2002) Enhanced emission and its switching in fluorescent organic nanoparticles. J Am Chem Soc 124(48):14410–14415
Li D, Li J, Duan Y, Zhao B, Ji B (2016) A highly efficient aggregation-induced emission fluorescent sensor for copper (ii) in aqueous media. Anal Methods 8(31):6013–6016
Pannipara M, Al-Sehemi AG, Kalam A, Asiri AM, Arshad MN (2017) AIE active turn-off fluorescent probe for the detection of Cu2+ ions. Spectrochim Acta Part A Mol Biomol Spectrosc 183:84–89
Assiri MA, Al-Sehemi AG, Pannipara M (2019) AIE based “on-off” fluorescence probe for the detection of Cu2+ ions in aqueous media. Inorg Chem Commun 99:11–15
Ghorai A, Mondal J, Manna AK, Chowdhury S, Patra GK (2018) A novel pyrene based highly selective reversible fluorescent-colorimetric sensor for the rapid detection of Cu 2+ ions: application in bio-imaging. Anal Methods 10(9):1063–1073
Wu SP, Huang ZM, Liu SR, Chung PK (2012) A pyrene-based highly selective turn-on fluorescent sensor for copper (II) ion and its application in live cell imaging. J Fluoresc 22(1):253–259
Venkatesan P, Wu SP (2015) A turn-on fluorescent pyrene-based chemosensor for Cu (II) with live cell application. RSC Adv 5(53):42591–42596
Kozlowski H, Janicka-Klos A, Brasun J, Gaggelli E, Valensin D, Valensin G (2009) Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation). Coord Chem Rev 253(21–22):2665–2685
Kumar M, Puri A (2012) A review of permissible limits of drinking water. Indian J Occup Environ Med 16(1):40
Ashish B, Neeti K, Himanshu K (2013) Copper toxicity: a comprehensive study. Res J Recent Sci 2(ISC-2012):58–67
Lutsenko S, Gupta A, Burkhead JL, Zuzel V (2008) Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch Biochem Biophys 476(1):22–32
Gupta VK, Ali I, Aboul-Enein HY (2007) Metal ions speciation in the environment: distribution, toxicities and analyses. Dev Environ Sci 5:33–56
Que EL, Domaille DW, Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 108(5):1517–1549
Gaggelli E, Kozlowski H, Valensin D, Valensin G (2006) Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem Rev 106(6):1995–2044
De Silva AP, Gunaratne HN, Gunnlaugsson T, Huxley AJ, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97(5):1515–1566
Brouwer AM (2011) Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl Chem 83(12):2213–2228
Nijegorodov NI, Downey WS (1994) The influence of planarity and rigidity on the absorption and fluorescence parameters and intersystem crossing rate constant in aromatic molecules. J Phys Chem 98(22):5639–5643
Qin W, Zhang P, Li H, Lam JW, Cai Y, Kwok RT, Tang BZ (2018) Ultrabright red AIEgens for two-photon vascular imaging with high resolution and deep penetration. Chem Sci 9(10):2705–2710
Chen J, Law CC, Lam JW, Dong Y, Lo SM, Williams ID, Tang BZ (2003) Synthesis, light emission, nanoaggregation, and restricted intramolecular rotation of 1, 1-substituted 2, 3, 4, 5-tetraphenylsiloles. Chem Mater 15(7):1535–1546
Hu R, Lager E, Aguilar-Aguilar A, Liu J, Lam JW, Sung HH, Tang BZ (2009) Twisted intramolecular charge transfer and aggregation-induced emission of BODIPY derivatives. J Phys Chem 113(36):15845–15853
Gruszecki WI (1991) Structural characterization of the aggregated forms of violaxanthin. J Biol Phys 18(2):99–109
Gu Z, Zhao M, Sheng Y, Bentolila LA, Tang Y (2011) Detection of mercury ion by infrared fluorescent protein and its hydrogel-based paper assay. Anal Chem 83(6):2324–2329
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, BaroneV,Petersson, GA, Nakatsuji H-Li-X,Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian, Inc., Gaussian 16, Revision B.01
Acknowledgements
P. K. Giri (Ref. No.: 228/(CSIR-UGC NET DEC 2017)) and S. S. Samanta (Award No.: 09/599(0084)/2019-EMR-I), and thank UGC and CSIR, New Delhi, India for their individual fellowship. Departmental instrumental facilities from DST FIST and UGC SAP programmes are gratefully acknowledged. We also acknowledge the help render by USIC, Vidyasagar University for doing both steady state and time resolved fluorescence study.
Funding
All the funders have been acknowledged in the acknowledgement section.
Author information
Authors and Affiliations
Contributions
All the authors (Prabhat Kumar Giri, Shashanka Sekhar Samanta, Naren Mudi, Milan Shyamal and Ajay Misra) made substantial contribution while preparing the manuscript and approve the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing Interest
The authors declare no competing financial interest
Software
A licensed version of Gaussian-16 software is used in computational study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Giri, P.K., Samanta, S.S., Mudi, N. et al. Highly Sensitive ‘on–off’ Pyrene Based AIEgen for Selective Sensing of Copper (II) Ions in Aqueous Media. J Fluoresc 32, 1059–1071 (2022). https://doi.org/10.1007/s10895-022-02929-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-02929-y