Abstract
Quinoxaline derivatives are well-known N-heterocycles with pharmacological and fluorescence activities. Almost all quinoxaline derivatives with extensive π-conjugation have been introduced as fluorophores which emit blue and green light. For the first time, we designed and synthesized 6-chloro-2,3 di(Pyridine-2yl) quinoxaline (2-CPQ) as a pink fluorophore in acetonitrile medium by simple route at room temperature whitin 30 min. The synthesized quinoxaline was identified using 1H, 13C NMR, MS, and FT-IR spectroscopy. Our results showed that the iodine-catalyzed method for both oxidation and cyclization during the synthesis of quinoxaline from pyridine 2-carbaldehyde was straightforward, efficient, and clean. All of the mentioned characterization devices confirmed the synthesis of 2-CPQ.
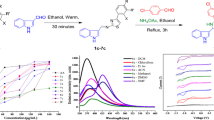
Moreover, we studied the photophysical properties of the synthesized fluorophore in which The UV–Vis absorption spectrum of 2-CPQ in DMF were three peaks at 451, 518 and 556 nm. Based on photophysical properties investigation, 2-CPQ shows good fluorescence with maximum peaks 607 and 653 nm in DMF as solvent (фF = 0.21). Hence, the fluorophore was applied in the peroxyoxalate chemiluminescence system. The reaction of imidazole, H2O2, and bis (2,4,6-trichlorophenyl) oxalate (TCPO) can transfer energy to a 6-chloro-2,3 di(pyridine-2yl) quinoxaline. In this process, dioxetane was synthesized, which chemically initiated the electron exchange luminescence (CIEEL) mechanism and led to pink light emission. We anticipate our synthesized fluorophores 2-CPQ will have great potential applications in imaging and medical markers.











Similar content being viewed by others
References
Pereira JA, Pessoa AM, Cordeiro MN, Fernandes R, Prudêncio C, Noronha JP, Vieira M (2015) Quinoxaline, its derivatives and applications: A State of the Art review. Eur J Med Chem 5:664–672. https://doi.org/10.1016/j.ejmech.2014.06.058
Sharma G, Abraham I, Tulsi P (2009) Synthesis of quinoxaline quinones and regioselectivity in their Diels-Alder cycloadditions. J Indian Chem 48:1590–1596
Nageswar YVD, Harsha Vardhan Reddy K, Ramesh K, Narayana Murthy S (2013) Recent developments in the synthesis of quinoxaline derivatives by green synthetic approaches. Org Prep Proced Int 45:1–27
Malik MA, Dar OA, Gull P, Wani MY, Hashmi AA (2018) Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. Med Chem Comm 9(3):409–436. https://doi.org/10.1039/C7MD00526A
Shamsi-Sani M, Shirini F, Abedini M, Seddighi M (2016) Synthesis of benzimidazole and quinoxaline derivatives using reusable sulfonated rice husk ash (RHA-SO3H) as a green and efficient solid acid catalyst. Res Chem Intermed 42:1091–1099. https://doi.org/10.1007/s11164-015-2075-5
Aparicio OA, Attanasi P, Filippone R, Ignacio S, Lillini F, Mantellini F, Palacios JM, Santos D (2006) Straightforward access to pyrazines, piperazinones, and quinoxalines by reactions of 1, 2-diaza-1, 3-butadienes with 1, 2-diamines under solution, solvent-free, or solid-phase conditions. J Org Chem 71(16):5897–5905. https://doi.org/10.1021/jo060450v
Kunkuma V, Bethala LAPD, Bhongiri Y, Rachapudi BNP, Potharaju SSP (2011) An efficient synthesis of quinoxalines catalyzed by monoammonium salt of 12-tungstophosphoric acid. Eur J Chem 2(4):495–498. https://doi.org/10.5155/eurjchem.2.4.495-498.413
Shi DQ, Ni SN, Shi JW, Dou GL, Li XY, Wang XS (2008) An efficient synthesis of polyhydroacridine derivatives by the three-component reaction of aldehydes, amines and dimedone in ionic liquid. J Heterocycl Chem 45(3):653–660. https://doi.org/10.1002/jhet.5570450303
Antoniotti S, Duñach E (2002) Direct and catalytic synthesis of quinoxaline derivatives from epoxides and ene-1, 2-diamines. Tetrahedron Lett 43(22):3971–3973
Abad N, Ramli Y, Ettahiri W, Ferfra S (2020) Quinoxaline derivatives: syntheses reactivities and biological properties. Moroccan J Heterocycl Chem. 19(2):1–62. https://doi.org/10.48369/IMIST.PRSM/jmch-v19i2.22352
Izadyar A, Omer KM, Liu Y, Chen S, Xu X, Bard AJ (2008) Electrochemistry and electrogenerated chemiluminescence of quinoxaline derivatives. J Phys Chem. 18;112 (50):20027–32. https://doi.org/10.1021/jp807202d
Qazvini NT, Zinatloo S (2011) Synthesis and characterization of gelatin nanoparticles using CDI/NHS as a non-toxic cross-linking system. J Mater Sci - Mater Med 22(1):63–6913. https://doi.org/10.1007/s10856-010-4178-2
Zinatloo AS, Taheri QN (2014) Inverse miniemulsion method for synthesis of gelatin nanoparticles in presence of CDI/NHS as a non-toxic cross-linking system. J Nanostruct 4(3):267–275. https://doi.org/10.1007/s10856-010-4178-2
Zinatloo-Ajabshir Z. Zinatloo-Ajabshir S (2019) Preparation and characterization of Curcumin niosomal nanoparticles via a simple and eco-friendly route. J Nanostruct 9(4):784–790. https://doi.org/10.22052/JNS.2019.04.020
Zinatloo-Ajabshir S, Mousavi-Kamazani M (2021) Recent advances in nanostructured Sn− Ln mixed-metal oxides as sunlight-activated nanophotocatalyst for high-efficient removal of environmental pollutants. Ceram Int 47:23702–23724. https://doi.org/10.1016/j.ceramint.2021.05.155
Zinatloo-Ajabshir S, Heidari-Asil SA, Salavati-Niasari M (2022) Rapid and green combustion synthesis of nanocomposites based on Zn–Co–O nanostructures as photocatalysts for enhanced degradation of acid brown 14 contaminant under sunlight. Sep Purif Technol 280:119841. https://doi.org/10.1016/j.seppur.2021.119841
Etemadi H, Afsharkia S, Zinatloo-Ajabshir S, Shokri E (2021) Effect of alumina nanoparticles on the antifouling properties of polycarbonate-polyurethane blend ultrafiltration membrane for water treatment. Polym Eng Sci 61(9):2364–2375
Kricka L (2000) Application of bioluminescence and chemiluminescence in biomedical sciences. Meth Enzymol 305:333–345. https://doi.org/10.1016/S0076-6879(00)05498-7Get
Yang L, Guan G, Wang S, Zhang Z (2012) Nano-anatase-enhanced peroxyoxalate chemiluminescence and its sensing application. J Phys Chem C 116(5):3356–3362. https://doi.org/10.1021/jp210316s
Kazemi SY, Abedirad SM, Vaezi Z, Ganjali MR (2012) A study of chemiluminescence characteristics of a novel peroxyoxalate system using berberine as the fluorophore. Dyes Pigm 95(3):751–756. https://doi.org/10.1016/j.dyepig.2012.05.022
Alvarez FJ, Parekh NJ, Matuszewski B, Givens RS, Higuchi T, Schowen R (1986) Multiple intermediates generate fluorophore-derived light in the oxalate/peroxide chemiluminescence system. J Am Chem Soc 108(20):6435–6437. https://doi.org/10.1021/ja00280a078
Stevani CV, Silva SM, Baader WJ (2000) Studies on the mechanism of the excitation step in peroxyoxalate Chemiluminescence. Eur J Org Chem 2000(24):4037–4046
Schuster GB (1979) Chemiluminescence of organic peroxides. Conversion of ground-state reactants to excited-state products by the chemically initiated electron-exchange luminescence mechanism. Acc Chem Res 12(10):366–373. https://doi.org/10.1021/ar50142a003
Ciscato LF, Bartoloni FH, Bastos EL, Baader WJ (2009) Direct kinetic observation of the chemiexcitation step in peroxyoxalate Chemiluminescence. J Org Chem 74(23):8974–8979. https://doi.org/10.1021/jo901402k
Shimakawa Y, Morikawa T, Sakaguchi S (2010) Facile route to benzils from aldehydes via NHC-catalyzed benzoin condensation under metal-free conditions. Tetrahedron Lett 51(13):1786–1789. https://doi.org/10.1016/j.tetlet.2010.01.103
18. Miyashita A, Suzuki Y, Iwamoto KI, Higashino T (1994) Catalytic action of azolium salts. VI. preparation of benzoins and acyloins by condensation of aldehydes catalyzed by azolium salts. Chem Pharm Bull 42(12):2633–2635. https://doi.org/10.1248/cpb.42.2633
Zhang Z, Xie C, Feng L, Ma C (2016) PTSA-catalyzed one-pot synthesis of quinoxalines using DMSO as the oxidant. Synth Commun 46(18):1507–1518. https://doi.org/10.1080/00397911.2016.1213297
Xie C, Zhang Z, Yang B, Song G, Gao H, Wen L, Ma C (2015) An efficient iodine–DMSO catalyzed synthesis of quinoxaline derivatives. Tetrahedron 71(12):1831–1837. https://doi.org/10.1016/j.tet.2015.02.003
De Jong GJ, Lammers N, Spruit FJ, Brinkman UT, Frei RW (1984) Optimization of a peroxyoxalate chemiluminescence detection system for the liquid chromatographic determination of fluorescent compounds. Chromatographia 18(3):129–133. https://doi.org/10.1007/BF02258768
Shamsipur M, Chaichi MJ, Karami AR (2003) A study of peroxyoxalate-chemiluminescence of acriflavine. Spectrochim Acta A Mol Biomol Spectrosc 59(3):511–517. https://doi.org/10.1016/S1386-1425(02)00188-9
Samadi-Maybodi A, Akhoondi R, Chaichi MJ (2010) Studies of New Peroxyoxalate-H 2 O 2 Chemiluminescence System Using Quinoxaline Derivatives as Green Fluorophores. J Fluoresc 20(3):671–679. https://doi.org/10.1007/s10895-010-0601-9
Martelo L, Periyasami G, Fedorov AA, Baleizao C, Berberan-Santos MN (2019) Chemiluminescence of naphthalene analogues of luminol in solution and micellar media. Dyes Pigm 168:341–346. https://doi.org/10.1016/j.dyepig.2019.05.005
Kazemi SY, Abedirad SM, Zali SH, Amiri M (2012) Hypericin from St. John’s Wort (hypericum perforatum) as a novel natural fluorophore for chemiluminescence reaction of bis (2, 4, 6-trichlorophenyl) oxalate–H2O2–imidazole and quenching effect of some natural lipophilic hydrogen peroxide scavengers. J Lumin 132(5):1226–1231. https://doi.org/10.1016/j.jlumin.2011.12.009
Givens RS, Schowen RL, Birks JW (1989) Chemiluminescence and photochemical reaction detection in chromatography. VCH, New York, USA ([Chapter 5])
Hadd AG, Seeber A, Birks JW (2000) Kinetics of two pathways in peroxyoxalate chemiluminescence. J Org Chem 65(9):2675–2683. https://doi.org/10.1021/jo9917487
Emteborg M, Pontén E, Irgum K (1997) Influence of imidazole and bis (trichlorophenyl) oxalate in the oxalyldiimidazole peroxyoxalate chemiluminescence reaction. Anal Chem 69(11):2109–2114. https://doi.org/10.1021/ac961225o
Stevani CV, de Arruda Campos IP, Baader WJ (1996) Synthesis and characterisation of an intermediate in the peroxyoxalate chemiluminescence: 4-chlorophenyl O, O-hydrogen monoperoxyoxalate. J Chem Soc Perkin Trans I 2(8):1645–1648. https://doi.org/10.1039/P29960001645
Dong J, Yang H, Li Y, Liu A, Wei W, Liu S (2020) Fluorescence sensor for organophosphorus pesticide detection based on the alkaline phosphatase-triggered reaction. Anal Chim Acta 1131:102–108. https://doi.org/10.1016/j.aca.2020.07.048
Wang L, Cui M, Tang H, Cao D (2018) Fluorescent nanoaggregates of quinoxaline derivatives for highly efficient and selective sensing of trace picric acid. Dyes Pigm 155:107–113. https://doi.org/10.1016/j.dyepig.2018.03.036
Yang W, Yang Y, Zhan L, Zheng K, Chen Z, Zeng X, Yang C (2020) Polymorphism-dependent thermally activated delayed fluorescence materials with diverse three dimensional supramolecular frameworks. Chem Eng J 390:124626. https://doi.org/10.1016/j.cej.2020.124626
Jonsson T, Irgum K (2000) New nucleophilic catalysts for bright and fast peroxyoxalate Chemiluminescence. Anal Chem 72(7):1373–1380. https://doi.org/10.1021/ac991339a
Emteborg M, Pontén E, Irgum K (1997) Influence of imidazole and bis (trichlorophenyl) oxalate in the oxalyldiimidazole peroxyoxalate chemiluminescence reaction. Anal Chem 558:69(11):2109–2114. https://doi.org/10.1021/ac961225o
Jonsson T, Emteborg M, Irgum K (1998) Heterocyclic compounds as catalysts in the peroxyoxalate chemiluminescence reaction of bis (2, 4, 6-trichlorophenyl) oxalate. Anal chim acta 361(3):205–215. https://doi.org/10.1016/S0003-2670(98)00029-4
Acknowledgements
This research was supported by a grant from the research council of Mazandaran University of Medical Sciences, Iran (No. 1161), this work dedicated to a part of Zahra Hashemi's post-doctoral project.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Zahra Hashemi was the first researcher, summarized the synthesis and characterization of 2-CPQ. Mohammad Ali Ebrahimzadeh was the principal investigator and designed the study. Pourya Biparva was the principal investigator and designed the analysis. Data analysis was performed by Seyed Mohammad Abedirad.
Corresponding authors
Ethics declarations
Conflicts of Interest
Not applicable.
Data Availability
The authors confirm that the findings and results of this study are available.
Code Availability
Not applicable.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hashemi, Z., Ebrahimzadeh, M.A., Biparva, P. et al. Pyridine-2-yl Quinoxaline (2-CPQ) Derivative As a Novel Pink Fluorophore: Synthesis, and Chemiluminescence Characteristics. J Fluoresc 32, 723–736 (2022). https://doi.org/10.1007/s10895-022-02890-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-02890-w