Abstract
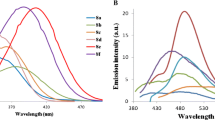
Fluorescein (1), a known fluorescent tracer in microscopy with high photophysical properties, was esterified to have fluorescein ethyl ester (2) and O-ethyl-fluorescein ethyl ester (3) in excellent yields. All of them were investigated for the photophysical and electrochemical properties as potential organic semiconductor materials. Absorptions and emission spectra were taken in various solvents, compound 2 showed emission maxima at λmax = 545 and compound 3 showed λmax = 550 nm. Optical band gap energy (Eg) was calculated for 1–3 and the values were found in between 2.34 – 2.39 eV. Possibility of shifting emission maxima was studied in various pH (5–9) buffers, and finally the thermal stability was examined using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Increasing of conjugation system of 2 and 3 were studied by HOMO and LUMO distributions of 1–3. Experimental results showed that compounds 2 and 3 have excellent photophysical and electrochemical properties hence can be used as excellent organic semiconductor materials.













Similar content being viewed by others
Data Availability
Unreported NMR, IR and Mass spectra for compounds 1–3 are given in supporting information files, samples and all other data and materials from this manuscript will be made available on request.
References
Prachumrak N, Namuangruk S, Keawin T, Jungsuttingwong S, Sudyoadsuk T, Promarak V (2013) Synthesis and Characterization of 9-(Fluoren-2-yl)anthracene Derivatives as Efficient Non-Doped Blue Emitters for Organic Light-Emitting Diodes. Eur J Org Chem 18:3825–3834. https://doi.org/10.1002/ejoc.201300165
Jürgensen N, Kretzschmar A, Höfle S, Freudenberg J, Bunz UHF, Hernandez-Sosa G (2017) Sulfone-Based Deep Blue Thermally Activated Delayed Fluorescence Emitters: Solution-Processed Organic Light-Emitting Diodes with High Efficiency and Brightness. Chem Mater 29(21):9154–9161. https://doi.org/10.1021/acs.chemmater.7b02964
Kalyani NT, Swart H, Dhoble SJ (2017) Chapter 2 - Luminescence in Organic Semiconductors. In: Kalyani NT, Swart H, Dhoble SJ (eds) Principles and Applications of Organic Light Emitting Diodes (OLEDs). Woodhead Publishing, pp 39–64. https://doi.org/10.1016/B978-0-08-101213-0.00002-3
Costa JCS, Taveira RJS, Lima CFRAC, Mendes A, Santos LMNBF (2016) Optical band gaps of organic semiconductor materials. Opt Mater 58:51–60. https://doi.org/10.1016/j.optmat.2016.03.041
Baeyer A (1871) Ueber eine neue Klasse von Farbstoffen. Ber Dtsch Chem Ges 4(2):555–558. https://doi.org/10.1002/cber.18710040209
Gessner T, Mayer U (2000) Triarylmethane and Diarylmethane Dyes. In: Ullmann's Encyclopedia of Industrial Chemistry. https://doi.org/10.1002/14356007.a27_179
Fluorescein (2021) https://en.wikipedia.org/wiki/Fluorescein#cite_note-Ull-1. Accessed 31/01/2021
Hentz NG, Richardson JM, Sportsman JR, Daijo J, Sittampalam GS (1997) Synthesis and characterization of insulin-fluorescein derivatives for bioanalytical applications. Anal Chem 69(24):4994–5000. https://doi.org/10.1021/ac970726m
Mills CO, Milkiewicz P, Molloy DP, Baxter DJ, Elias E (1997) Synthesis, physical and biological properties of lithocholyl-lysyl-fluorescein: a fluorescent monohydroxy bile salt analogue with cholestatic properties. Biochim Biophys Acta 20(3):485–496. https://doi.org/10.1016/s0304-4165(97)00063-9
Onishi H, Machida Y (1999) Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials 20(2):175–182. https://doi.org/10.1016/s0142-9612(98)00159-8
Yamana K, Mitsui T, Nakano H (1999) Synthesis and properties of oligonucleotide duplexes containing donor and acceptor fluorophores at 2′-positions. Tetrahedron 55(30):9143–9150. https://doi.org/10.1016/S0040-4020(99)00484-6
Dias CS, Mitra AK (2000) Vitreal elimination kinetics of large molecular weight FITC-labeled dextrans in albino rabbits using a novel microsampling technique. J Pharm Sci 89(5):572–578. https://doi.org/10.1002/(SICI)1520-6017(200005)89:5%3c572::AID-JPS2%3e3.0.CO;2-P
Hattori S, Fujisaki H, Kiriyama T, Yokoyama T, Irie S (2002) Real-time zymography and reverse zymography: a method for detecting activities of matrix metalloproteinases and their inhibitors using FITC-labeled collagen and casein as substrates. Anal Biochem 301(1):27–34. https://doi.org/10.1006/abio.2001.5479
Heyder P, Gaipl US, Beyer TD, Voll RE, Kern PM, Stach C, Kalden JR, Herrmann M (2003) Early detection of apoptosis by staining of acid-treated apoptotic cells with FITC-labeled lectin from Narcissus pseudonarcissus. Cytometry A 55(2):86–93. https://doi.org/10.1002/cyto.a.10078
Metallo SJ, Kane RS, Holmlin RE, Whitesides GM (2003) Using bifunctional polymers presenting vancomycin and fluorescein groups to direct anti-fluorescein antibodies to self-assembled monolayers presenting d-alanine-d-alanine groups. J Am Chem Soc 125(15):4534–4540. https://doi.org/10.1021/ja030045a
Nakayama H, Arakaki A, Maruyama K, Takeyama H, Matsunaga T (2003) Single-nucleotide polymorphism analysis using fluorescence resonance energy transfer between DNA-labeling fluorophore, fluorescein isothiocyanate, and DNA intercalator, POPO-3, on bacterial magnetic particles. Biotechnol Bioeng 84(1):96–102. https://doi.org/10.1002/bit.10755
Teranishi K, Nishiguchi T (2004) Cyclodextrin-bound 6-(4-methoxyphenyl)imidazo[1,2-alpha+/-]pyrazin-3(7H)-ones with fluorescein as green chemiluminescent probes for superoxide anions. Anal Biochem 325(2):185–195. https://doi.org/10.1016/j.ab.2003.10.042
Liu M, Xie C, Pan H, Pan J, Lu W (2006) Separation of polyethylene glycols and their fluorescein-labeled compounds depending on the hydrophobic interaction by high-performance liquid chromatography. J Chromatogr A 29(1):61–66. https://doi.org/10.1016/j.chroma.2006.06.088
Hoffmann C, Leroy-Dudal J, Patel S, Gallet O, Pauthe E (2008) Fluorescein isothiocyanate-labeled human plasma fibronectin in extracellular matrix remodeling. Anal Biochem 372(1):62–71. https://doi.org/10.1016/j.ab.2007.07.027
Cho HK, Lone S, Kim DD, Choi JH, Choi SW, Cho JH, Kim JH, Cheong IW (2009) Synthesis and characterization of fluorescein isothiocyanate (FITC)-labeled PEO–PCL–PEO triblock copolymers for topical delivery. Polymer 50(11):2357–2364. https://doi.org/10.1016/j.polymer.2009.03.032
Liu JM, Huang XM, Liu ZB, Lin SQ, Li FM, Gao F, Li ZM, Zeng LQ, Li LY, Ouyang Y (2009) Exploitation of phosphorescent labelling reagent of fullerol-fluorescein isothiocyanate and new method for the determination of trace alkaline phosphatase as well as forecast of human diseases. Anal Chim Acta 648(2):226–234. https://doi.org/10.1016/j.aca.2009.06.041
Puckett CA, Barton JK (2009) Fluorescein redirects a ruthenium-octaarginine conjugate to the nucleus. J Am Chem Soc 131(25):8738–8739. https://doi.org/10.1021/ja9025165
Brush CK (1994) Fluorescein labelled phosphoramidites. US Patent US005583236A, December 10, 1996
Weatherman RV, Kiessling LL (1996) Fluorescence Anisotropy Assays Reveal Affinities of C- and O-Glycosides for Concanavalin A(1). J Org Chem 61(2):534–538. https://doi.org/10.1021/jo951430o
Adamczyk M, Chan CM, Fino JR, Mattingly PG (2000) Synthesis of 5- and 6-hydroxymethylfluorescein phosphoramidites. J Org Chem 65(2):596–601. https://doi.org/10.1021/jo991449h
Natarajan A, Moerke N, Fan YH, Chen H, Christ WJ, Wagner G, Halperin JA (2004) Synthesis of fluorescein labeled 7-methylguanosinemonophosphate. Bioorg Med Chem Lett 14(10):2657–2660. https://doi.org/10.1016/j.bmcl.2004.02.090
Singleton SF, Roca AI, Lee AM, Xiao J (2007) Probing the structure of RecA–DNA filaments. Advantages of a fluorescent guanine analog. Tetrahedron 63 (17):3553–3566. https://doi.org/10.1016/j.tet.2006.10.092
Unciti-Broceta A, Diezmann F, Ou-Yang CY, Fara MA, Bradley M (2009) Synthesis, penetrability and intracellular targeting of fluorescein-tagged peptoids and peptide-peptoid hybrids. Bioorg Med Chem 17(3):959–966. https://doi.org/10.1016/j.bmc.2008.02.068
Marchand P, Becerril-Ortega J, Mony L, Bouteiller C, Paoletti P, Nicole O, Barré L, Buisson A, Perrio C (2011) Confocal Microscopy Imaging of NR2B-Containing NMDA Receptors Based on Fluorescent Ifenprodil-Like Conjugates. Bioconjug Chem 23(1):21–26. https://doi.org/10.1021/bc100571g
Kierat RM, Kramer R (2005) A fluorogenic and chromogenic probe that detects the esterase activity of trace copper(II). Bioorg Med Chem Lett 15(21):4824–4827. https://doi.org/10.1016/j.bmcl.2005.07.042
Crisp GT, Millan MJ (1998) Conjugate addition of amino acid side chains to dyes containing alkynone, alkynoic ester and alkynoic amide linker arms. Tetrahedron 54(3–4):649–666. https://doi.org/10.1016/S0040-4020(97)10324-6
Wang H, Li J, Liu X, Zhang H-S (2000) N-hydroxysuccinimidyl fluorescein-O-acetate as a highly fluorescent derivatizing reagent for aliphatic amines in liquid chromatography. Anal Chim Acta 423(1):77–83. https://doi.org/10.1016/S0003-2670(00)01097-7
Jing B, Zhu D (2004) Fullerene–fluorescein–anthracene hybrids: a model for artificial photosynthesis and solar energy conversion. Tetrahedron Lett 45(1):221–224. https://doi.org/10.1016/j.tetlet.2003.10.136
Uthirakumar P, Suh E-K, Hong C-H, Lee Y-S (2005) Synthesis and characterization of polyesters containing fluorescein dye units. Polymer 46(13):4640–4646. https://doi.org/10.1016/j.polymer.2005.03.063
Chen X, Ma H (2006) A selective fluorescence-on reaction of spiro form fluorescein hydrazide with Cu(II). Anal Chim Acta 575(2):217–222. https://doi.org/10.1016/j.aca.2006.05.097
Ge FY, Chen LG, Zhou XL, Pan HY, Yan FY, Bai GY, Yan XL (2007) Synthesis and study on hydrolytic properties of fluorescein esters. Dyes Pigm 72(3):322–326. https://doi.org/10.1016/j.dyepig.2005.09.012
Chen X, Li Z, Xiang Y, Tong A (2008) Salicylaldehyde fluorescein hydrazone: a colorimetric logic chemosensor for pH and Cu(II). Tetrahedron Lett 49(32):4697–4700. https://doi.org/10.1016/j.tetlet.2008.05.137
Du XL, Zhang HS, Deng YH, Wang H (2008) Design and synthesis of a novel fluorescent reagent, 6-oxy-(ethylpiperazine)-9-(2’-methoxycarbonyl) fluorescein, for carboxylic acids and its application in food samples using high-performance liquid chromatography. J Chromatogr A 1178(1–2):92–100. https://doi.org/10.1016/j.chroma.2007.11.047
Eshghi H, Mirzaie N, Asoodeh A (2011) Synthesis of fluorescein aromatic esters in the presence of P2O5/SiO2 as catalyst and their hydrolysis studies in the presence of lipase. Dyes Pigm 89(2):120–126. https://doi.org/10.1016/j.dyepig.2010.09.013
Li T, Yang Z, Li Y, Liu Z, Qi G, Wang B (2011) A novel fluorescein derivative as a colorimetric chemosensor for detecting copper(II) ion. Dyes Pigm 88(1):103–108. https://doi.org/10.1016/j.dyepig.2010.05.008
Ying LQ, Branchaud BP (2011) Purification of tetracysteine-tagged proteins by affinity chromatography using a non-fluorescent, photochemically stable bisarsenical affinity ligand. Bioconjug Chem 22(5):987–992
Zhang L, Zhang X (2014) A selectively fluorescein-based colorimetric probe for detecting copper(II) ion. Spectrochim Acta A Mol Biomol Spectrosc 133:54–59. https://doi.org/10.1016/j.saa.2014.04.130
Fujita S, Toru T, Kitagawa Y, Kagiyama N, Momiyama M (1997) Highly sensitive detection of membrane-bound DNA using fluorescein derivatives. Anal Chim Acta 339(3):289–296. https://doi.org/10.1016/S0003-2670(96)00479-5
Adamczyk M, Mattingly PG, Moore JA (1998) O-(fluoresceinylmethyl) hydroxylamine (OFMHA): a fluorescent reagent for detection of damaged nucleic acids. Bioorg Med Chem Lett 8(24):3599–3602. https://doi.org/10.1016/s0960-894x(98)00658-1
Rukavishnikov AV, Zaikova TO, Birrell GB, Keana JF, Griffith OH (1999) Synthesis of a new fluorogenic substrate for the continuous assay of mammalian phosphoinositide-specific phospholipase C. Bioorg Med Chem Lett 9(8):1133–1136. https://doi.org/10.1016/s0960-894x(99)00166-3
Mugherli L, Burchak ON, Chatelain F, Balakirev MY (2006) Fluorogenic ester substrates to assess proteolytic activity. Bioorg Med Chem Lett 16(17):4488–4491. https://doi.org/10.1016/j.bmcl.2006.06.037
Feng S, Gong S, Feng G (2020) Aggregation-induced emission and solid fluorescence of fluorescein derivatives. Chem Commun 56(16):2511–2513. https://doi.org/10.1039/C9CC09784H
Oliveira E, Bértolo E, Núñez C, Pilla V, Santos HM, Fernández-Lodeiro J, Fernández-Lodeiro A, Djafari J, Capelo JL, Lodeiro C (2018) Green and Red Fluorescent Dyes for Translational Applications in Imaging and Sensing Analytes: A Dual-Color Flag 7(1):9–52. https://doi.org/10.1002/open.201700135
Ilican S, Caglar M, Caglar Y (2007) Determination of the thickness and optical constants of transparent indium-doped ZnO thin films by the envelope method. Mater Sci-Pol 25 (3):709–718. https://materialsscience.pwr.edu.pl/bi/vol25no3/articles/ms_15ilic.pdf
Bhadwal AS, Tripathi RM, Gupta RK, Kumar N, Singh RP, Shrivastav A (2014) Biogenic synthesis and photocatalytic activity of CdS nanoparticles. RSC Adv 4(19):9484–9490. https://doi.org/10.1039/C3RA46221H
Ganapuram BR, Alle M, Dadigala R, Dasari A, Maragoni V, Guttena V (2015) Catalytic reduction of methylene blue and Congo red dyes using green synthesized gold nanoparticles capped by salmalia malabarica gum. International Nano Letters 5(4):215–222. https://doi.org/10.1007/s40089-015-0158-3
Lohse J, Nielsen PE, Harrit N, Dahl O (1997) Fluorescein-Conjugated Lysine Monomers for Solid Phase Synthesis of Fluorescent Peptides and PNA Oligomers. Bioconjug Chem 8(4):503–509. https://doi.org/10.1021/bc9700704
Rahman AFMM, Park S-E, Kadi AA, Kwon Y (2014) Fluorescein Hydrazones as Novel Nonintercalative Topoisomerase Catalytic Inhibitors with Low DNA Toxicity. J Med Chem 57(21):9139–9151. https://doi.org/10.1021/jm501263m
Islam MS, Park S, Song C, Kadi AA, Kwon Y, Rahman AFMM (2017) Fluorescein hydrazones: A series of novel non-intercalative topoisomerase IIα catalytic inhibitors induce G1 arrest and apoptosis in breast and colon cancer cells. Eur J Med Chem 125:49–67. https://doi.org/10.1016/j.ejmech.2016.09.004
Muhammad FF, Abdul Hapip AI, Sulaiman K (2010) Study of optoelectronic energy bands and molecular energy levels of tris (8-hydroxyquinolinate) gallium and aluminum organometallic materials from their spectroscopic and electrochemical analysis. J Organomet Chem 695(23):2526–2531. https://doi.org/10.1016/j.jorganchem.2010.07.026
Duvenhage MM, Visser HG, Ntwaeaborwa OM, Swart HC (2014) The effect of electron donating and withdrawing groups on the morphology and optical properties of Alq3. Physica B 439:46–49. https://doi.org/10.1016/j.physb.2013.11.049
Blanksby SJ, Ellison GB (2003) Bond dissociation energies of organic molecules. Acc Chem Res 36(4):255–263. https://doi.org/10.1021/ar020230d
da Silva G, Chen C-C, Bozzelli JW (2006) Bond dissociation energy of the phenol OH bond from ab initio calculations. Chem Phys Lett 424(1):42–45. https://doi.org/10.1016/j.cplett.2006.04.022
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RG-1436-024.
Funding
This research was funded by the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RG-1436–024.
Author information
Authors and Affiliations
Contributions
G.A.E. Mostafa performed absorbance and fluorescence measurements; A.S. Mahajumi checked the literature, wrote the part of manuscript, H. AlRabiah and A.A. Kadi provided the equipment’s and laboratory facilities, revised the manuscript; Y. Lu performed HOMO–LUMO calculation and wrote the part of manuscript; A.F.M.M. Rahman conceived the research thought, designed the experimental scheme, edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no conflict of interest/competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mostafa, G.A.E., Mahajumi, A.S., AlRabiah, H. et al. Synthesis and Photophysical Properties of Fluorescein Esters as Potential Organic Semiconductor Materials. J Fluoresc 31, 1489–1502 (2021). https://doi.org/10.1007/s10895-021-02789-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02789-y