Abstract
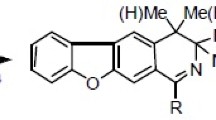
A convenient synthetic approach to asymmetrically functionalized 1,3-di(2-pyridyl)benzenes starting from 3-(3-bromophenyl)-1,2,4-triazines using sequential aza-Diels–Alder reactions and Stille cross-coupling is reported. Photophysical properties of the obtained compounds are studied.





Similar content being viewed by others
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author (Igor L. Nikonov) upon reasonable request.
Code Availability
Legally obtained software was used for the manuscript preparation and for the data collection and analysis.
References
I-Jen C, Yun C, Tai-Nan D, Hsiao-Yun K, Bi-Hai T (2015) Heteroleptic Ir(III) phosphors with bis-tridentate chelating architecture for high efficiency OLEDs. US Patent 9,219,237 B1.
Tasuku S, Norio S, Wataru S, Tasuku S, Norio S (2007) Organometallic Complex, Luminescent Solid, Organic el Element and Organic el Display. US Patent 2007/0224447 A1.
Jae JB, Young JS, Joon KH, Hoon LD, Young LJ, Hwan SC (2013) New heterocyclic compounds and organic electronic device using the same. KR Patent 10-2013-0135178 A.
Tong B, Ku H-Y, Chen I-J, Chi Y, Kao H-C, Yeh C-C, Chang C-H, Liu S-H, Lee G-H, Chou P-T (2015) Heteroleptic Ir(III) phosphors with bis-tridentatechelating architecture for high efficiency OLEDs. J Mater Chem C 3(14):3460–3471. https://doi.org/10.1039/C5TC00163C
Wang Z, Turner E, Mahoney V, Madakuni S, Groy T, Li J (2010) Facile synthesis and characterization of phosphorescent Pt(N∧C∧N)X complexes. Inorg Chem 49(24):11276–11286. https://doi.org/10.1021/ic100740e
Kui SCF, Chow PK, Tong GSM, Lai S-L, Cheng G, Kwok C-C, Low K-H, Ko MY, Che C-M (2013) Robust phosphorescent platinum(II) complexes containing tetradentate O^N^C^N ligands: excimeric excited state and application in organic white-light-emitting diodes. Chem Eur J 19(1):69–73. https://doi.org/10.1002/chem.201203687
Cinninger LM, Bastatas LD, Shen Y, Holliday BJ, Slinker JD (2019) Luminescent properties of a 3,5-diphenylpyrazolebridged Pt(II) dimer. Dalton Trans 48(26):9684–9691. https://doi.org/10.1039/C9DT00795D
Liu S, Zhang G, Lu J, Jia J, Li W, Huang F, Cao Y (2015) An alcohol soluble amino-functionalized organoplatinum(II) complex as the cathode interlayer for highly efficient polymer solar cells. J Mater Chem C 3(17):4372–4379. https://doi.org/10.1039/C5TC00452G
Katagiri S, Sakamoto R, Maeda H, Nishimori Y, Kurita T, Nishihara H (2013) Terminal Redox-Site effect on the long-range electron conduction of Fe(tpy)2 oligomer wires on a gold electrode. Chem Eur J 19(16):5088–5096. https://doi.org/10.1002/chem.201203913
Chen Y, Lu W, Che C-M (2013) Luminescent pincer-type cyclometalated Platinum(II) complexes with auxiliary isocyanide ligands: phase-transfer preparation, solvatomorphism, and self-aggregation. Organometallics 32(1):350–353. https://doi.org/10.1021/om300965b
Vezzu DAK, Lu Q, Chen Y-H, Huo S (2014) Cytotoxicity of cyclometalated platinum complexes based on tridentate NCN and CNN-coordinating ligands: Remarkable coordination dependence. J Inorg Biochem 134:49–56. https://doi.org/10.1016/j.jinorgbio.2014.01.021
Shi H, Clarkson GJ, Sadler PJ (2019) Dual action photosensitive Platinum(II) anticancer prodrugs with photoreleasable azide ligands. Inorg Chim Acta 489:230–235. https://doi.org/10.1016/j.ica.2019.02.016
Dyker G, Gabler M, Nouroozian M, Schulz P (1994) Isoquinolines as receptors for resorcinol. Tetrahedron Lett 35(52):9697–9700. https://doi.org/10.1016/0040-4039(94)88362-9
Bönnemann H (1978) Cobalt-catalyzed pyridine syntheses from alkynes and nitriles. Angew Chem Int Ed Engl 17:505–515. https://doi.org/10.1002/anie.197805051
Biller SA, Sofia MJ, DeLange B, Forster C, Gordon EM, Harrity T, Rich LC, Ciosek CP Jr (1991) The first potent inhibitor of squalene synthase: a profound contribution of an ether oxygen to inhibitor-enzyme interaction. J Am Chem Soc 113:8522–8524. https://doi.org/10.1021/ja00022a050
Allan KM, Hong BD, Stoltz BM (2009) Expedient synthesis of 3-hydroxyisoquinolines and 2-hydroxy-1,4-naphthoquinones via one-pot aryne acyl-alkylation/condensation. Org Biomol Chem 7:4960–4964. https://doi.org/10.1039/B913336D
Gildea LF, Batsanov AS, Williams JAG (2013) Bright orange/red-emitting Rhodium(III) and Iridium(III) complexes: tridentate N^C^N-cyclometallating ligands lead to high luminescence efficiencies. Dalton Trans 42:10388–10393. https://doi.org/10.1039/C3DT51211H
Kozhevnikov VN, Donnio B, Bruce DW (2008) Phosphorescent, terdentate, liquid-crystalline complexes of Platinum(II): stimulus-dependent emission. Angew Chem Int Ed 47(33):6286–6289. https://doi.org/10.1002/anie.200802101
Pfueller OC, Sauer J (1998) The new and simple ‘LEGO’ system for the synthesis of thienyl substituted 2,6-oligopyridines. Tetrahedron Lett 39(48):8821–8824. https://doi.org/10.1016/S0040-4039(98)02043-7
Stanforth SP, Tarbit B, Watson MD (2003) Synthesis of 2,2′-bipyridyl derivatives using aza Diels-Alder methodology. Tetrahedron Lett 44(4):693–694. https://doi.org/10.1016/S0040-4039(02)02670-9
Prokhorov AM, Kozhevnikov DN (2012) Reactions of triazines and tetrazines with dienophiles (Review). Chem Heterocycl Compd 48(8):1153–1176. https://doi.org/10.1007/s10593-012-1117-9
Kopchuk DS, Nikonov IL, Khasanov AF, Gundala S, Krinochkin AP, Slepukhin PA, Zyryanov GV, Venkatapuram P, Chupakhin ON, Charushin VN (2019) One-step synthesis of 1,4-bis(het)arylisoquinolines by the reaction of 1,2,4-triazines with arynes. Chem Heterocycl Compd 55(10):978–984. https://doi.org/10.1007/s10593-019-02565-8
Kopchuk DS, Khasanov AF, Kovalev IS, Zyryanov GV, Kim GA, Nikonov IL, Rusinov VL, Chupakhin ON (2014) The extension of conjugated system in pyridyl-substituted monoazatriphenylenes for the tuning of photophysical properties. Chem Heterocycl Compd 50(6):871–879. https://doi.org/10.1007/2Fs10593-014-1541-0
Shabunina OV, Yu KD, Krinochkin AP, Kim GA, Kopchuk DS, Zyryanov GV, Fi L, Chupakhin ON (2017) π-Extended fluorophores based on 5-aryl-2,2’-bipyridines: synthesis and photophysical studies. Mendeleev Commun 27(6):602–604. https://doi.org/10.1016/j.mencom.2017.11.021
Starnovskaya ES, Kopchuk DS, Khasanov AF, Tanya OS, Santra S, Giri K, Rahman M, Kovalev IS, Zyryanov GV, Majeed A, Charushin VN (2019) Synthesis and photophysics of new unsymmetrically substituted 5,5′-diaryl-2,2′-bypiridine-based “push-pull” fluorophores. Dyes Pigm 162:324–330. https://doi.org/10.1016/j.dyepig.2018.10.040
Kopchuk DS, Chepchugov NV, Starnovskaya ES, Khasanov AF, Krinochkin AP, Santra S, Zyryanov GV, Das P, Majee A, Rusinov VL, Charushin VN (2019) Synthesis and optical properties of new 2-(5-arylpyridine-2-yl)-6-(het)arylquinoline-based “push-pull” fluorophores. Dyes Pigm 167:151–156. https://doi.org/10.1016/j.dyepig.2019.04.029
Chupakhin ON, Rusinov VL, Ulomsky EN, Kojevnikov DN, Neunhoeffer H (1997) Nucleophilic substitution of hydrogen in the reaction of 1,2,4-triazine-4-oxides with cyanides. Mendeleev Commun 7(2):66–67. https://doi.org/10.1070/MC1997v007n02ABEH000700
Kozhevnikov VN, Kozhevnikov DN, Nikitina TV, Rusinov VL, Chupakhin ON, Zabel M, König B (2003) A versatile strategy for the synthesis of functionalized 2,2‘-bi- and 2,2‘:6‘,2‘‘-terpyridines via their 1,2,4-triazine analogues. J Org Chem 68(7):2882–2888. https://doi.org/10.1021/jo0267955
Charushin VN, Chupakhin ON (2019) Nucleophilic C—H functionalization of arenes: a contribution to green chemistry. Russ Chem Bull, Int Ed 68:453–471. https://doi.org/10.1007/s11172-019-2441-3
Kozhevnikov DN, Kozhevnikov VN, Rusinov VL, Chupakhin ON (1997) A general method for the synthesis of 1,2,4-triazine 4-oxides. Mendeleev Commun 7(6):238. https://doi.org/10.1070/MC1997v007n06ABEH000875
Saraswathi TV, Srinivasan VR (1977) Syntheses and spectral characteristics of 6-mono-, 3,6-di- and 3,5,6-trisubstituted-1,2,4-triazines. Tetrahedron 33(9):1043–1051. https://doi.org/10.1016/0040-4020(77)80223-8
Kopchuk DS, Kovalev IS, Zyryanov GV, Khasanov AF, Medvedevskikh AS, Rusinov VL, Chupakhin ON (2013) Phenylglyoxal dihydrazones as unexpected products in the synthesis of 1,2,4-triazines by interaction of α-bromoacetophenones and arylhydrazides. Chem Heterocycl Compd 49(7):988–992. https://doi.org/10.1007/2Fs10593-013-1336-8
Kozhevnikov VN, Ustinova MM, Slepukhin PA, Santoro A, Bruce DW, Kozhevnikov DN (2008) From 1,2,4-triazines towards substituted pyridines and their cyclometallated Pt complexes. Tetrahedron Lett 49(26):4096–4098. https://doi.org/10.1016/j.tetlet.2008.04.138
Gers CF, Nordmann J, Kumru C, Frank W, Müller TJJ (2014) Solvatochromic fluorescent 2-substituted 3-ethynyl quinoxalines: four-component synthesis, photophysical properties, and electronic structure. J Org Chem 79(8):3296–3310. https://doi.org/10.1021/jo4025978
Wang K, Huang S, Zhang Y, Zhao S, Zhang H, Wang Y (2013) Multicolor fluorescence and electroluminescence of an ICT-type organic solid tuned by modulating the accepting nature of the central core. Chem Sci 4(8):3288–3293. https://doi.org/10.1039/C3SC51091C
Nisic F, Colombo A, Dragonetti C, Fontani M, Marinotto D, Righetto S, Roberto D, Williams JAG (2015) Highly efficient acido-triggered reversible luminescent and nonlinear optical switch based on 5-π-delocalized-donor-1,3-di(2-pyridyl)benzenes. J Mater Chem C 3(28):7421–7427. https://doi.org/10.1039/C5TC01529D
Li N, Lai S-L, Liu W, Wang P, You J, Lee C-S, Liu Z (2011) Synthesis and properties of n-type triphenylpyridine derivatives and applications in deep-blue organic light-emitting devices as electron-transporting layer. J Mater Chem 21(34):12977–12985. https://doi.org/10.1039/C1JM11898F
Li Y-J, Sasabe H, Su S-J, Tanaka D, Takeda T, Pu Y-J, Kido J (2009) Phenanthroline derivatives for electron-transport layer in organic light-emitting devices. Chem Lett 38(7):712–713. https://doi.org/10.1246/cl.2009.712
Tan S, Wu X, Zheng Y, Wang Y (2019) Synthesis and properties of novel N, C, N terdentate skeleton based on 1,3-di(pyridin-2-yl)benzene moiety-new tricks for old dogs. Chin Chem Lett 30(11):1951–1954. https://doi.org/10.1016/j.cclet.2019.08.003
Yang X, Liu Y, Li J, Wang Q, Yang M, Li C (2018) A novel aggregation-induced-emission-active supramolecular organogel for the detection of volatile acid vapors. New J Chem 42(21):17524–17532. https://doi.org/10.1039/C8NJ02616E
Kanimozhi R, Singh FV (2020) Metal-free synthesis and characterization of 1,3-Bis(heteroaryl)benzenes followed by the photophysical studies using ultraviolet–visible and fluorescence spectroscopy. J Mol Struct 1219:128633.
Porrès L, Holland A, Pålsson L-O, Monkman AP, Kemp C, Beeby A (2006) Absolute measurements of photoluminescence quantum yields of solutions using an integrating sphere. J Fluoresc 16(2):267–272. https://doi.org/10.1007/s10895-005-0054-8
Kopchuk DS, Chepchugov NV, Taniya OS, Khasanov AF, Giri K, Kovalev IS, Santra S, Zyryanov GV, Majee A, Rusinov VL, Chupakhin ON (2016) 3-Cyano-2-azaanthracene-based “push-pull” fluorophores: A one-step preparation from 5-cyano-1,2,4-triazines and 2,3-dehydronaphthalene, generated in situ. Tetrahedron Lett 57(50):5639–5643. https://doi.org/10.1016/j.tetlet.2016.11.008
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer, Boston
Acknowledgements
This work was supported by Russian Science Foundation (Grant # 19-73-10144) and Grants Council of the President of the Russian Federation (no. NSh-2700.2020.3).
Funding
This work was supported by Russian Science Foundation (Grant # 19–73-10,144) and Grants Council of the President of the Russian Federation (no. NSh-2700.2020.3). No other sources of funding were involved.
Author information
Authors and Affiliations
Contributions
Ekaterina S. Starnovskaya: synthesis of the ligands, drafting the manuscript, final version approval; Dmitry S. Kopchuk: concept design, drafting the manuscript, final version approval; Yaroslav K. Shtaitz: synthesis of the ligands, drafting the manuscript, final version approval; Maria I. Savchuk: synthesis of the ligands and ptotophysical experiments, drafting the manuscript, final version approval; Igor L. Nikonov: ptotophysical experiments, drafting the manuscript, final version approval; Ilya N. Egorov: concept design, analysis of the literature data, rafting the manuscript, final version approval; Grigory V. Zyryanov: concept design, analysis of the obtained results, rafting the manuscript, final version approval; Vladimir L. Rusinov: concept design, analysis of the obtained results, drafting the manuscript, final version approval; Oleg N. Chupakhin: concept design, analysis of the obtained results, drafting the manuscript, final version approval.
Corresponding author
Ethics declarations
Ethical Approval
This manuscript was not be submitted to more than one journal for simultaneous consideration.
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Starnovskaya, E.S., Kopchuk, D.S., Shtaitz, Y.K. et al. Asymmetrically Functionalized 1,3-Di(2-pyridyl)benzenes: Synthesis and Photophysical Studies. J Fluoresc 32, 125–133 (2022). https://doi.org/10.1007/s10895-021-02759-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02759-4