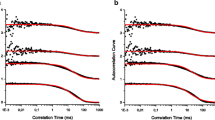
Abstract
Fluorescence Correlation Spectroscopy (FCS) studies of the interaction of polymers or proteins in solution are strongly affected by the viscosity and refractive index of the medium, and the effects are likely to be more significant with the use of short wavelength excitation (e.g., 405 nm diode lasers). Failing to account for these issues can lead to incorrect measurement of average size, conformational changes, and dynamic behaviour of polymers and proteins. Steady-state, time-resolved, and FCS measurements of Alexa 405 in glycerol:water mixtures were performed to determine its suitability for FCS measurements with 405 nm excitation. The effects of the refractive index and viscosity on the diffusion coefficient and photophysical parameters (lifetime and relative quantum yield) of the fluorophore were determined. Alexa 405 lifetime decreased from 3.55 ns in water to 3.25 ns in a 50:50 glycerol:water mixture, while its diffusion coefficient dropped from 333 ± 16 to 44 ± 1 µm2s− 1. Lifetime data collected from micromolar solutions of Alexa 405 did however also suggest that as solvent polarity decreased, aggregates (excimers) were formed as evidenced by the appearance of a rising edge in the decay plots. The interdependence between lifetime, refractive index, and diffusion coefficient could be accurately fitted by a simple polynomial function indicating that the probe is well behaved and predictable in the glycerol:water model system. Overall, Alexa 405 is a most promising and reliable probe for FCS measurement using violet laser diode excitation sources.




Similar content being viewed by others
Data Availability
Data will be made available on request.
Code Availability
Not applicable.
Notes
We do not have the capability to measure RI at 422 nm.
References
Balbo J, Mereghetti P, Herten DP, Wade RC (2013) The shape of protein crowders is a major determinant of protein diffusion. Biophys J 104(7):1576–1584. https://doi.org/10.1016/j.bpj.2013.02.041
Pack CG, Nishimura G, Tamura M, Aoki K, Taguchi H, Yoshida M, Kinjo M (1999) Analysis of interaction between chaperonin GroEL and its substrate using fluorescence correlation spectroscopy. Cytometry 36(3):247–253
Nolan R, Iliopoulou M, Alvarez L, Padilla-Parra S (2018) Detecting protein aggregation and interaction in live cells: A guide to number and brightness. Methods 140–141:172–177. https://doi.org/10.1016/j.ymeth.2017.12.001
Rezgui R, Blumer K, Yeoh-Tan G, Trexler AJ, Magzoub M (2016) Precise quantification of cellular uptake of cell-penetrating peptides using fluorescence-activated cell sorting and fluorescence correlation spectroscopy. Biochim Biophys Acta 1858(7 Pt A):1499–1506. https://doi.org/10.1016/j.bbamem.2016.03.023
Mittag JJ, Milani S, Walsh DM, Radler JO, McManus JJ (2014) Simultaneous measurement of a range of particle sizes during Abeta1-42 fibrillogenesis quantified using fluorescence correlation spectroscopy. Biochem Biophys Res Commun 448(2):195–199. https://doi.org/10.1016/j.bbrc.2014.04.088
Wang F, Shi Y, Luo S, Chen Y, Zhao J (2012) Conformational transition of Poly(N-isopropylacrylamide) single chains in its cononsolvency process: a study by fluorescence correlation spectroscopy and scaling analysis. Macromolecules 45(22):9196–9204. https://doi.org/10.1021/ma301780f
Dominguez-Medina S, Chen S, Blankenburg J, Swanglap P, Landes CF, Link S (2016) Measuring the hydrodynamic size of nanoparticles using fluctuation correlation spectroscopy. Annu Rev Phys Chem 67:489–514. https://doi.org/10.1146/annurev-physchem-040214-121510
Cedervall T, Lynch I, Foy M, Berggard T, Donnelly SC, Cagney G, Linse S, Dawson KA (2007) Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew Chem 46(30):5754–5756. https://doi.org/10.1002/anie.200700465
Watanabe C, Yanagisawa M (2018) Cell-size confinement effect on protein diffusion in crowded poly(ethylene)glycol solution. Phys Chem Chem Phys 20(13):8842–8847. https://doi.org/10.1039/c7cp08199e
Haidekker MA, Ling T, Anglo M, Stevens HY, Frangos JA, Theodorakis EA (2001) New fluorescent probes for the measurement of cell membrane viscosity. Chem Biol 8:123–131
Weissberg SG, Simha R, Rothman S (1951) Viscosity of dilute and moderately concentrated polymer solutions. J Res Natl Bur Stand 47:298–314
Wöll D (2014) Fluorescence correlation spectroscopy in polymer science. RSC Adv 4(5):2447–2465
Ota C, Noguchi S, Nagatoishi S, Tsumoto K (2016) Assessment of the protein-protein interactions in a highly concentrated antibody solution by using Raman spectroscopy. Pharm Res 33(4):956–969. https://doi.org/10.1007/s11095-015-1842-8
Ota C, Noguchi S, Tsumoto K (2015) The molecular interaction of a protein in highly concentrated solution investigated by Raman spectroscopy. Biopolymers 103(4):237–246. https://doi.org/10.1002/bip.22593
Garidel P, Pevestorf B, Bahrenburg S (2015) Stability of buffer-free freeze-dried formulations: A feasibility study of a monoclonal antibody at high protein concentrations. Eur J Pharm Biopharm 97:125–139. https://doi.org/10.1016/j.ejpb.2015.09.017
Szasz C, Alexa A, Toth K, Rakacs M, Langowski J, Tompa P (2011) Protein disorder prevails under crowded conditions. Biochemistry 50(26):5834–5844. https://doi.org/10.1021/bi200365J
Enderlein J, Gregor I, Patra D, Dertinger T, Kaupp UB (2005) Performance of fluorescence correlation spectroscopy for measuring diffusion and concentration. Chemphyschem 6(11):2324–2336. https://doi.org/10.1002/cphc.200500414
Chattopadhyay K, Saffarian S, Elson EL, Frieden C (2005) Measuring unfolding of proteins in the presence of denaturant using fluorescence correlation spectroscopy. Biophys J 88(2):1413–1422. https://doi.org/10.1529/biophysj.104.053199
Lehmann S, Seiffert S, Richtering W (2015) Refractive index mismatch can misindicate anomalous diffusion in single-focus fluorescence correlation spectroscopy. Macromol Chem Phys 216(2):156–163. https://doi.org/10.1002/macp.201400349
Banachowicz E, Patkowski A, Meier G, Klamecka K, Gapinski J (2014) Successful FCS experiment in nonstandard conditions. Langmuir 30(29):8945–8955. https://doi.org/10.1021/la5015708
Wang S, Arena ET, Eliceiri KW, Yuan M (2018) Automated and robust quantification of colocalization in dual-color fluorescence microscopy: a nonparametric statistical approach. IEEE Trans Image Process 27(2):622–636
Chung HS, Meng F, Kim JY, McHale K, Gopich IV, Louis JM (2017) Oligomerization of the tetramerization domain of p53 probed by two- and three-color single-molecule FRET. PNAS 114(33):E6812–E6821
Chung HS, McHale K, Louis JM, Eaton WA (2012) Single-molecule fluorescence experiments determine protein folding transition path times. Science 335:981–984
Szczurek AT, Prakash K, Lee H-K, Żurek-Biesiada DJ, Best G, Hagmann M, Dobrucki JW, Cremer C, Birk U (2014) Single molecule localization microscopy of the distribution of chromatin using Hoechst and DAPI fluorescent probes. Nucleus 5(4):331–334-
Girkin JM, Ferguson AI, Wokosin DL, Gurney AM (2000) Confocal microscopy using an InGaN violet laser diode at 406 nm. Opt Express 7(10):336–41
Luo D, Kuang C, Liu X, Wang G (2013) Experimental investigations on fluorescence excitation and depletion of ATTO 390 dye. Opt Laser Technol 45:723–725. https://doi.org/10.1016/j.optlastec.2012.05.003
Ruttinger S, Kramer VB,B, Erdmann R, Macdonald R, Koberling F (2008) Comparison and accuracy of methods to determine the confocal volume for quantitative fluorescence correlation spectroscopy. J Microsc 232(2):343–352
Resch-Genger U, Rurack K (2013) Determination of the photoluminescence quantum yield of dilute dye solutions (IUPAC Technical Report). Pure Appl Chem 85(10):2005–2013. https://doi.org/10.1351/pac-rep-12-03-03
Muller P, Schwille P, Weidemann T (2014) PyCorrFit-generic data evaluation for fluorescence correlation spectroscopy. Bioinformatics 30(17):2532–2533. https://doi.org/10.1093/bioinformatics/btu328
Strickler SJ, Berg RA (1962) Relationship between absorption intensity and fluorescence lifetime of molecules. J Chem Phys 37(4):814–822. https://doi.org/10.1063/1.1733166
Toptygin D, Savtchenko RS, Meadow ND, Roseman S, Brand L (2002) Effect of the solvent refractive index on the excited-state lifetime of a single tryptophan residue in a protein. J Phys Chem B 106(14):3724–3734
Fisher TR, Zhou G, Shi Y, Huang L (2020) How does hydrogen bond network analysis reveal the golden ratio of water–glycerol mixtures? PCCP 22(5):2887–2907. https://doi.org/10.1039/C9CP06246G
Song D, Yang R, Fang SY, Liu YP, Long F (2018) A FRET-based dual-color evanescent wave optical fiberaptasensor for simultaneous fluorometric determination of aflatoxin M1 and ochratoxin A. Microchim Acta 185(11):10. https://doi.org/10.1007/s00604-018-3046-5
Tanahashi T, Kawaguchi M, Honda T, Takahashi A (1994) Adsorption of poly(N-isopropylacrylamide) on silica surfaces. Macromolecules 27(2):606–607. https://doi.org/10.1021/ma00080a040
Callewaert M, Grandfils C, Boulangé-Petermann L, Rouxhet PG (2004) Adsorption of poly(N-isopropylacrylamide) on glass substrata. J Colloid Interface Sci 276(2):299–305. https://doi.org/10.1016/j.jcis.2004.03.063
Gao J, Hu TJ, Zhang YB, Li P, Wu C (1999) The adsorption of linear poly(N-isopropylacrylamide) chains on surfactant-free polystyrene nanoparticles. Chin J Polym Sci 17(6):595–601
Meaney PM, Fox CJ, Geimer SD, Paulsen KD (2017) Electrical characterization of glycerin: water mixtures: implications for use as a coupling medium in microwave tomography. IEEE Trans Microw Theory Tech 65(5):1471–1478
Quinn GEM Jr, Litovitz RG (1962) Dielectric properties of glycerol—water mixtures. J Chem Phys 37(2):239–242. https://doi.org/10.1063/1.1701311
Racknor C, Singh MR, Zhang Y, Birch DJS, Chen Y (2014) Energy transfer between a biological labelling dye and gold nanorods. Methods and Applications in Fluorescence 2(1)6. https://doi.org/10.1088/2050-6120/2/1/015002
Krishna MMG (1999) Excited-state kinetics of the hydrophobic probe nile red in membranes and micelles. J Phys Chem A 103(19):3589–3595
Behrends R, Fuchs K, Kaatze U, Hayashi Y, Feldman Y (2006) Dielectric properties of glycerol/water mixtures at temperatures between 10 and 50°C. J Chem Phys 124:144512
Rurack K, Spieles M (2011) Fluorescence quantum yields of a series of red and near-infrared dyes emitting at 600–1000 nm. Anal Chem 83(4):1232–1242. https://doi.org/10.1021/ac101329h
Casamayou-Boucau Y, Ryder AG (2018) Accurate anisotropy recovery from fluorophore mixtures using Multivariate Curve Resolution (MCR). Anal Chim Acta 1000:132–143. https://doi.org/10.1016/j.aca.2017.11.031
Freire S, de Araujo MH, Al-Soufi W, Novo M (2014) Photophysical study of Thioflavin T as fluorescence marker of amyloid fibrils. Dyes Pigm 110:97–105. https://doi.org/10.1016/j.dyepig.2014.05.004
Gafni A, Brand L (1976) Fluorescence decay studies of reduced nicotinamide adenine dinucleotide in solution and bound to liver alcohol dehydrogenase. Biochemistry 15(15):3165–3171
Gaiduk A, Ruijgrok PV, Yorulmaz M, Orrit M (2011) Making gold nanoparticles fluorescent for simultaneous absorption and fluorescence detection on the single particle level. Phys Chem Chem Phys 13:149–153
Chattopadhyay PK, Gaylord B, Palmer A, Jiang N, Raven MA, Geoff L, Reuter MA, Rahman AKM, Price N, Betts DA, Roederer MR (2012) Brilliant violet fluorophores: a new class of ultrabright fluorescent compounds for immunofluorescence experiments. Cytometry Part A 81A:456–466
Telford W, Kapoor V, Jackson J, Burgess W, Buller G, Hawley T, Hawley R (2006) Violet laser diodes in flow cytometry: an update. Cytometry A 69(11):1153–1160. https://doi.org/10.1002/cyto.a.20340
Stefl M, Benda A, Gregor I, Hof M (2014) The fast polarization modulation based dual-focus fluorescence correlation spectroscopy. Opt Express 22(1):885–899. https://doi.org/10.1364/OE.22.000885
Muller P, Schwille, P, Weidemann T (2014) PyCorrFit—generic data evaluation for fluorescence correlation spectroscopy. Bioinforma Appl Note 30(17):2532–2533
Müller CB, Loman A, Pacheco V, Koberling F, Willbold D, Richtering W, Enderlein J (2008) Precise measurement of diffusion by multi-color dual-focus fluorescence correlation spectroscopy. EPL 83(4):46001. https://doi.org/10.1209/0295-5075/83/46001
Diaspro A, Federici F, Robello M (2002) Influence of Refractive Index Mismatch in high-resolution three-dimensional confocal microscopy. Appl Opt 41(4):685–690
Meseth U, Wohland T, Rigler R, Vogel H (1999) Resolution of fluorescence correlation measurements. Biophys J 76:1619–1631
Volk A CJK (2018) Density model for aqueous glycerol solutions. Exp Fluids 59(5). article number 75, https://doi.org/10.1007/s00348-018-2527-y
Cook RL, King HE Jr, Herbst CA, Herschbach DR (1994) Pressure and temperature dependent viscosity of two glass forming liquids: Glycerol and dibutyl phthalate. J Chem Phys 100(7):5178–5189. https://doi.org/10.1063/1.467276
Ferreira AGM, Egas APV, Fonseca IMA, Costa AC, Abreu DC, Lobo LQ (2017) The viscosity of glycerol. J Chem Thermodyn 113:162–182. https://doi.org/10.1016/j.jct.2017.05.042
Armarego WLF, Chai CLL (2003) Purification of laboratory chemicals, 5th edn. Butterworth-Heinemann, Oxford
Acknowledgements
This publication has emanated from research supported in part by a research grant from CAPES (Grant No. BEX 1415-15-8) and from Science Foundation Ireland (SFI) which was co-funded under the European Regional Development Fund under Grant number (14/IA/2282, Advanced Analytics for Biological Therapeutic Manufacture, to AGR). The FCS instrumentation was provided under an Irish Health Research Board Equipment Grant (EQ/2004/29).
Funding
This publication has emanated from research supported in part by a research grant from CAPES (Grant No. BEX 1415-15-8 to CVZ) and from Science Foundation Ireland (SFI) which was co-funded under the European Regional Development Fund under Grant number (14/IA/2282, Advanced Analytics for Biological Therapeutic Manufacture, to AGR). The FCS instrumentation was provided under an Irish Health Research Board Equipment Grant (EQ/2004/29).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
The authors have no conflicting or competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 425 KB)
Rights and permissions
About this article
Cite this article
van Zanten, C., Melnikau, D. & Ryder, A.G. Effects of Viscosity and Refractive Index on the Emission and Diffusion Properties of Alexa Fluor 405 Using Fluorescence Correlation and Lifetime Spectroscopies. J Fluoresc 31, 835–845 (2021). https://doi.org/10.1007/s10895-021-02719-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02719-y