Abstract
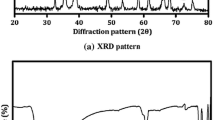
To investigate the effect of Gd x SrO: CdO (x = 0.1, 0.3, 0.4) nanostructures (NS), in the present work an attempt has been made to synthesize Gdx SrO:CdO NS by co precipitation method. Structural properties were investigated by XRD (X-ray diffraction), FTIR (Fourier transform infrared spectroscopy), SEM (scanning electron microscopy), UV-Visible, XPS (X-ray photoelectron spectroscopy). XRD indicates having mixed phase of tetragonal crystal structure and SEM images indicate spherical shaped nanoparticles (NPs) of Gd x SrO:CdO with average size laying in between ~100 nm to ~130 nm. FTIR spectra of Gd x SrO: CdO NS show stretching and bending peaks of Gd-O-Gd, Cd-O-Cd and Sr-OH at ~1311 cm −1, ~1486 cm −1, ~ 3300 cm −1 and UV-visible optical absorptivity of Gd x SrO:CdO show absorption maxima shift from 330 nm to 324 nm (blue shift) and edges at 352.4 nm, 348 nm and 346.3 nm respectively for Gd concentration varying between 0.1, 0.3 and 0.4. binding energies of the Gd 3d 3/2, Sr 3d 3/2 and Cd 3d 3/2, O1s and C1s observed at 150.8 eV, 141.6 eV, 410.1 eV, 529.6 eV and 282.4 eV respectively which confirms the chemical composition of NS. Photoluminescence (PL) spectrum of Gd 0.4 Sr 0.5 O Cd 0.1O NS exhibit broad peaks from 338 nm to 397 nm centred around 369 nm with various Gd, O, Sr and Cd related native defects. Emission band observed at UV- Visible region for Gd 0.3 Sr 0.5 O Cd 0.2 O NS PL emission spectra has two emission peaks at 369 nm (UV region) and 550 nm (Visible region). The transitions can be ascertained with shielding of 4f shells of Gd+3 ions by 6 s, 5d shells by the interaction of the other Gd+3 ions.












Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article [supplementary information files].
References
Guo H, Li Y, Wang D, Zhang W, Yin M, Lou L (2004) Blue upconversion of cubic Gd2O3: Er produced by green laser. J Alloys Compd 376:23–27. https://doi.org/10.1016/j.jallcom.2003.12.020
Lushchik A, Savikhin F, Tokbergenov I (2003) Electron and hole intraband luminescence in complex metal oxides. J Lumin 102-103:44–47. https://doi.org/10.1016/S0022-2313(02)00530-6
Yang CT, Padmanabhan P, Gulyas BZ (2016) Gadolinium(iii) based nanoparticles for T1-weighted magnetic resonance imaging probes. RSC Adv 6:60945–60966. https://doi.org/10.1039/C6RA07782J
Tamrakar RK, Bisen DP, Brahme N (2014) Comparison of photoluminescence properties of Gd2O3 phosphor synthesized by combustion and solid-state reaction method. J Radiat Res Appl 7:550–559. https://doi.org/10.1016/j.jrras.2014.09.005
Shankar R, Srivastava RK (2018) Photoconductivity and luminescence properties of gadolinium doped zinc oxide. India Sect A Phys Sci 88:137–144. https://doi.org/10.1007/s40010-016-0328-3
Aggarwal N, Kaur K, Vasishth A, Verma NK (2016) Structural, optical and magnetic properties of gadolinium-doped ZnO nanoparticles. J Mater Sci Mater Electron 27:12–13011. https://doi.org/10.1007/s10854-016-5440-2
Zhang N, Chen D, Niu F, Wang S, Qin L, Huang Y (2016) Enhanced visible light photocatalytic activity of Gd-doped BiFeO3 nanoparticles and mechanism insight. Sci Rep 6:26467. https://doi.org/10.1038/srep26467
Paul S, Choudhury B, Choudhury A (2014) Magnetic property study of Gd doped TiO2 nanoparticles. J Alloys Compd 601:201–206. https://doi.org/10.1016/j.jallcom.2014.02.070
Thambidurai M, Muthukumarasamy N, Velauthapillai D, Lee C (2013) Quantum confinement effects in Gd-doped CdS nanoparticles prepared by chemical precipitation technique. J Mater Sci Mater Electron 24:4535–4541. https://doi.org/10.1007/s10854-013-1438-1
Smyntyna V, Semenenko B, Skobeeva V, Malushin N (2014) Photoactivation of luminescence in CdS nanocrystals. Beilstein J Nanotechnol 5:355–359. https://doi.org/10.3762/bjnano.5.40
Kaur K, Lotey GS, Verma NK (2014) Structural, magnetic, dielectric and magnetodielectric properties of Gd-doped CdS nanorods. Mater Sci Semicond Process 19:6–10. https://doi.org/10.1016/j.mssp.2013.11.021
Murmu PP, Mendelsberg RJ, Kennedy J, Carder DA, Ruck BJ, Markwitz A, Reeves RJ, Malar P, Osipowicz T (2011) Structural and photoluminescence properties of Gd implanted ZnO single crystals. J Appl Phys 110:033534. https://doi.org/10.1063/1.3619852
Soumya RD, Singh AK, Deshmukh L, Gupta A (2015) Structural, morphological and optical studies on chemically deposited nanocrystalline Gd-doped Cd0.5Zn0.5Se thin films. Opt quant Electron. https://doi.org/10.1007/s11082-014-0093-y
Singh V, Sivaramaiah G, Singh N, Pathaka MS, Rao JL, Jirimalid HD, Natarajane V (2018) Investigation of ultraviolet emitting Gd doped Sr2MgSi2O7 phosphors. Optik 169:397–402. https://doi.org/10.1016/j.ijleo.2018.05.036
Gong N, Wang H, Li S, Deng Y, Chen X, Ye L, Wei G (2014) Microwave-assisted Polyol synthesis of gadolinium-doped green luminescent carbon dots as a bimodal Nanoprobe. Langmuir 30:10933–10939. https://doi.org/10.1021/la502705g
Selvaraju C, Karthick R, Veerasubam R (2019) The modification of structural, optical and antibacterial activity properties of rare earth gadolinium-doped ZnO nanoparticles prepared by co-precipitation method. J Inorg Organomet Polym Mater 29:776–782. https://doi.org/10.1007/s10904-018-1051-0
Park CW, JinSeo HJ (2014) Self-activated luminescence characteristics of double perovskite ceramic Sr2ZrCeO6. Ceram Int 40:2495–2499. https://doi.org/10.1016/j.ceramint.2013.07.061
Das R, Gupta K, Jana K, Nayak A, Ghosh UC (2016) Preparation, characterization and dielectric, ac conductivity with electrochemical behavior of strontium zirconate. Adv Mater Lett 7:646–651. https://doi.org/10.5185/amlett.2016.6294
Mishra S, Khare A, Tiwari S, Kshatri DS (2016) Diminution in photoluminescent intensity of SrS: Ce3þ phosphor due to increased milling time. J Alloys Compd 695:1956–1965. https://doi.org/10.1016/j.jallcom.2016.11.030
Zhang H, Zhao S, Wang X, Ren X, Ye J, Huang L, Xu S (2019) The enhanced photoluminescence and temperature sensing performance in rare earth doped SrMoO4 phosphors by aliovalent doping: from material design to device applications. J Mater Chem C 7:15007. https://doi.org/10.1039/c9tc04965g
Chawla P, Locha SP, Singh N (2011) Synthesis and luminescence studies of CdSrS nanostructures. J Alloys Compd 509:72–75. https://doi.org/10.1016/j.jallcom.2010.08.084
Firdous A, Ahmad MM (2012) Optical and High- Temperature Electrical Properties of pure and Sr-modified CdS Nanocrystals. Int J Nanosci 11:1250009. https://doi.org/10.1142/S0219581X12500093
Ahmed A, Siddique NM, Alam U, Ali T, Tripathi P (2018) Improved Photocatalytic activity of Sr doped SnO2 nanoparticles: a role of oxygen vacancy. Appl Surf Sci 463:976–985. https://doi.org/10.1016/j.apsusc.2018.08.182
Vanga PR, Mangalaraja RV, Ashok M (2016) Effect of co-doping on the optical, magnetic and photocatalytic properties of the Gd modified BiFeO3. J Mater Sci Mater Electron 27:5699–5706. https://doi.org/10.1007/s10854-016-4481-x
Morales AE, Mora ES, Pal U (2007) Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev Mex de Fis 53:18 https://www.redalyc.org/articulo.oa?id=57028299004
Aggarwal N, Kaur K, Vasishth A, Verma NK (2016) Structural optical and magnetic properties of gadolinium-doped ZnO nanoparticles. J Mater Sci Mater Electron 27:13006–13011. https://doi.org/10.1007/s10854-016-5440-2
Sahu D, Panda NR, Acharya BS (2017) Effect of Gd doping on structure and photoluminescence properties of ZnO nanocrystals. Mater Res Express 4:114001. https://doi.org/10.1088/2053-1591/aa9597/meta
Vijayaprasth D, Murugan R, Hayakawa Y, Ravi G, Lumin J (2016) Optical and magnetic studies on Gd doped ZnO nanoparticles synthesized by co-precipitation method. J Lumin 178:375–383. https://doi.org/10.1016/j.jlumin.2016.06.004
Gupta SK, Kadam RM, Natarajan V, Godbole SV (2014) Nanoparticles of Sr0.995Gd0.005ZrO3-gel-combustion synthesis, characterization, fluorescence and EPR spectroscopy. Mater Sci Eng B 183:6–11. https://doi.org/10.1016/j.mseb.2013.11.024
Adhikari R, Das AK, Karmakar D, Ghatak J (2010) Gd-doped SnO2 nanoparticles: structure and magnetism, J. Magn Magn 322:3631–3637. https://doi.org/10.1016/j.jmmm.2010.07.006
Huang K, Yu J, Zhang L, Xu J, Yang Z, Liu C, Wang W, Kan X (2019) Structural and magnetic properties of GdeZn substituted M-type BaeSr hexaferrites by sol-gel auto-combustion method. J Alloys Compd 803:971–980. https://doi.org/10.1016/j.jallcom.2019.06.348
Zhao X, Wang W, Zhang Y, Wu S, Li F, Liu JP (2014) Synthesis and characterization of gadolinium doped cobalt ferrite nanoparticles with enhanced adsorption capability for Congo red. Int J Chem Eng 250:164–174. https://doi.org/10.1016/j.cej.2014.03.113
Jadhav LD, Chourashiya MG, Subhedar KM, Tyagi AK, Patil JY (2009) Synthesis of nanocrystalline Gd doped ceria by combustion technique. J Alloys Compd 470:383–386. https://doi.org/10.1016/j.jallcom.2008.02.077
Dubey V, Agrawal S, Kaur J (2014) Photoluminescence and thermoluminescence behaviour of Gd doped Y2O3 phosphor. Optik 126:1–5. https://doi.org/10.1016/j.ijleo.2014.06.175
Paulraj K, Ramaswamy S, Arulanantham AMS, Valanarasu S, Shkir M, Ganesh V, AlFaify S, Kim H, Kathalingam A (2019) Investigation on nebulizer spray deposited Gd-doped PbS thin films for photo sensing applications. J Mater Sci: Mater 30:18858–18865. https://doi.org/10.1007/s10854-019-02242-8
Ibrahim IM (2019) Enhancement the sensitivity of CdS nano structure by adding of rare earth materials. J Phys Conf Ser 1178:20–21. https://doi.org/10.1088/1742-6596/1178/1/012013
Buscaglia MT, Maglia F, Anselmi-Tamburini U, Marré D, Pallecchi I, Ianculescu A, Canu G, Viviani M, Fabrizio M, Buscaglia V (2014) Effect of nanostructure on the thermal conductivity of La-doped SrTiO3 ceramics. J Eur Ceram 34:307–316. https://doi.org/10.1016/j.jeurceramsoc.2013.08.009
Im J, Park I, Shin D (2011) Electrochemical properties of nanostructured lanthanum strontium manganite cathode fabricated by electrostatic spray deposition. Solid State Ionics 192:448–452. https://doi.org/10.1016/j.ssi.2010.05.050
Zarkov A, Stanulis A, Mikoliunaite L, Katelnikovas A, Jasulaitiene V, Ramanauskas R, Tautkus S, Kareiva A (2017) Chemical solution deposition of pure and Gd-doped ceria thin films: structural, morphological and optical properties. Ceram Int 43:4280–4287. https://doi.org/10.1016/j.ceramint.2016.12.070
Anishur Rahman ATM, Vasilev K, Majewski P (2011) Ultra small Gd2O3 nanoparticles: absorption and emission properties. J Colloid Interface Sci 354:592–596. https://doi.org/10.1016/j.jcis.2010.11.012
Wang TX, Chen WW (2008) Solid phase preparation of submicron-sized SrTiO3 crystallites from SrO2 nanoparticles and TiO2 powders. Mater Lett 62:2865–2867. https://doi.org/10.1016/j.matlet.2008.01.062
Anishur Rahman ATM, Majewski P, Vasilev K (2013) Gd2O3 nanoparticles: size-dependent nuclear magnetic resonance. Contrast Media Mol Imaging 8:92–95. https://doi.org/10.1002/cmmi.1481
Rahman MM, Hussaina MM, Asiria AM (2017) Ultrasensitive and label-free detection of creatine based on CdO nanoparticles: a real sample approach. New J Chem 41:6667–6677. https://doi.org/10.1039/c6nj04101a
Gowri S, Gopinath K, Arumugam A (2018) Experimental and computational assessment of mycosynthesized CdO nanoparticles towards biomedical applications. J Photochem Photobiol 180:166–174. https://doi.org/10.1016/j.jphotobiol.2018.02.009
Rani S, Lal B, Saxena S, Shukla SH (2017) Photoluminescence properties of Gd:ZnO nano phosphor. J Sol-Gel Sci Technol 81:586–592. https://doi.org/10.1007/s10971-016-4218-6
Vijayaprasth G, Murugan R, Hayakawa Y, Ravi G Optical and magnetic studies on Gd doped ZnO nanoparticles synthesized by co-precipitation method. J Lumin 178:375–383. https://doi.org/10.1016/j.jlumin.2016.06.004
Cheemadan S, Rafiudeen A, Kumar MCS (2016) Highly transparent conducting CdO thin films by radiofrequency magnetron sputtering for optoelectronic applications. J Nanophotonics 10:033007–033007. https://doi.org/10.1117/1.JNP.10.033007
Ueda N, Maeda H, Hosono H, Kawazoe H (1998) Band-gap widening of CdO thin films. J Appl Phys 84:6174–6177. https://doi.org/10.1063/1.368933
Acknowledgements
Authors are thankful to IIT Kanpur for SEM and XPS analysis, Ranichennamma University for XRD and elemental compositional analysis, KUD, Dharwar for SEM EDX analysis.
Funding
Authors do not have received any funding from any institutions or agencies.
Author information
Authors and Affiliations
Contributions
Dr. Vinayak Adimule and Dr. Debdas Bhowmik contributed synthesis, manuscript preparation and communication. Dr. Yallur BC involved in characterization of the samples, Mr. Santosh S Nandi acknowledged for SEM, EDX and XRD analysis and partly by manuscript preparation. Mr. Adarsha H.J. Gowda carried out PL experimentation and interpretation of the results.
Corresponding authors
Ethics declarations
Conflict of Interests
All the authors declare that they do not have any competing interest.
Code Availability
“Not Applicable”.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 793 kb)
Rights and permissions
About this article
Cite this article
Adimule, V., Nandi, S.S., Yallur, B.C. et al. Optical, Structural and Photoluminescence Properties of Gd x SrO: CdO Nanostructures Synthesized by Co Precipitation Method. J Fluoresc 31, 487–499 (2021). https://doi.org/10.1007/s10895-021-02683-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02683-7