Abstract
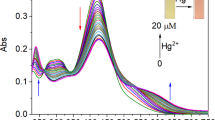
The highly selective and sensitive fluorometric method has been developed for trace level determination of Hg(II) is based on photo-induced electron transfer between rhodamine-6G dye and metal complex. Quenching in fluorescence intensity by fluorescence resonance energy transfer (FRET) is due to interaction between metal ion complex and dye. The fluorescence emitted was measured at 510 and 550 nm, for excitation and emission wavelengths respectively. Possible interferences present in water samples, which could affect the analytical response are studied and determined. The calibration graph was dynamically linear from 0.002 to 0.05 mgL−1 of Hg(II) with limit of detection 7 × 10−4 mgL−1 and limit of quantitation 1.9 × 10−3 mgL−1. The Stern-Volmer constant (KSV) calculated for the quenching of R-6G with Hg (II) was 8.47 Lmg−1 s−1 at optimized reaction conditions. The proposed FRET based fluorometric method was applied successfully in different industrial wastewater samples with satisfactory outcome.








Similar content being viewed by others
References
Bi N, Chen Y, Qi H, Zheng X, Chen Y, Liao X, Tian Y (2012) Spectrophotometric determination of mercury (II) ion using gold nanorod as probe. Sens Actuators B Chem 166:766–771. https://doi.org/10.1016/j.snb.2012.03.068
Huang D, Liu X, Lai C, Qin L, Zhang C, Yi H, Zhang Y (2019) Colorimetric determination of mercury (II) using gold nanoparticles and double ligand exchange. Microchim Acta 186:1–8. https://doi.org/10.1007/s00604-018-3126-6
Liu Y, Chang X, Hu X, Guo Y, Meng WF (2005) Highly selective determination of total mercury (II) sub microgram per liter by β-cyclodextrin polymer solid-phase spectrophotometry using 1, 3-di-(4-nitrodiazoamino)-benzene. Anal Chim Acta 532:121–128. https://doi.org/10.1016/j.aca.2004.10.062
Satnami ML, Vaishanav SK, Nagwanshi R, Ghosh KK (2016) Spectrofluorometric determination of mercury and lead by colloidal CdS nanomaterial. J Dispers Sci Technol 37:196–204. https://doi.org/10.1080/01932691.2015.1039020
Shamsipur M, Hosseini M, Alizadeh K, Alizadeh N, Yari A, Caltagirone C, Lippolis V (2005) Novel fluorimetric bulk optode membrane based on a dansylamidopropyl pendant arm derivative of 1-aza-4, 10-dithia-7-oxacyclododecane ([12] aneNS2O) for selective subnanomolar detection of Hg (II) ions. Anal Chim Acta 533:17–24. https://doi.org/10.1016/j.aca.2004.10.069
Alizadeh N, Moemeni A, Shamsipur M (2002) Poly (vinyl chloride)-membrane ion-selective bulk optode based on 1, 10-dibenzyl-1, 10-diaza-18-crown-6 and 1-(2-pyridylazo)-2-naphthol for Cu2+and Pb2+ ions. Anal Chim Acta 464:187–196. https://doi.org/10.1016/S0003-2670(02)00477-4
Rezaei B, Shahshahanipour M, Ensafi AA, Farrokhpour H (2017) Development of highly selective and sensitive fluorimetric label-free mercury aptasensor based on cysteamine@ CdTe/ZnS quantum dots, experimental and theoretical investigation. Sens Actuators B Chem 247:400–407. https://doi.org/10.1016/j.snb.2017.03.082
Yang YK, Yook KJ, Tae J (2005) A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J Am Chem Soc 127:16760–16761. https://doi.org/10.1021/ja054855t
Niazi A, Momeni-Isfahani AZ (2009) Spectrophotometric determination of mercury in water samples after cloud point extraction using nonionic surfactant triton X-114. J Hazard Mater 165:1200–1203. https://doi.org/10.1016/j.jhazmat.2008.09.091
De Wuilloud JC, Wuilloud RG, Silva MF, Olsina RA, Martinez LD (2002) Sensitive determination of mercury in tap water by cloud point extraction pre-concentration and flow injection-cold vapor-inductively coupled plasma optical emission spectrometry. Spectrochim Acta Part B 57:365–374. https://doi.org/10.1016/S0584-8547(01)00393-7
Fong BMW, Siu TS, Lee JSK, Tam S (2007) Determination of mercury in whole blood and urine by inductively coupled plasma mass spectrometry. J Anal Toxicol 31:281–287. https://doi.org/10.1093/jat/31.5.281
Voegborlo RB, Akagi H (2007) Determination of mercury in fish by cold vapour atomic absorption spectrometry using an automatic mercury analyzer. Food Chem 100:853–858. https://doi.org/10.1016/j.foodchem.2005.09.025
Çaylak O, Elçi ŞG, Höl A, Akdoğan A, Divrikli Ü, Elçi L (2019) Use of an aminated Amberlite XAD-4 column coupled to flow injection cold vapour generation atomic absorption spectrometry for mercury speciation in water and fish tissue samples. Food Chem 274:487–493. https://doi.org/10.1016/j.foodchem.2018.08.107
Leao DJ, Junior MMS, Junior JBS, de Oliveira DA, Queiroz AF, Ferreira SL (2016) Ultrasound assisted extraction for the determination of mercury in sediment samples employing cold vapour atomic absorption spectrometry. Anal Methods 8:6554–6559. https://doi.org/10.1039/C6AY01810F
Giacomino A, Redda AR, Squadrone S, Rizzi M, Abete MC, La Gioia C, Malandrino M (2017) Anodic stripping voltammetry with gold electrodes as an alternative method for the routine determination of mercury in fish. Comparison with spectroscopic approaches. Food Chem 221:737–745. https://doi.org/10.1016/j.foodchem.2016.11.111
Kamyabi MA, Aghaei A (2016) Electromembrane extraction and anodic stripping voltammetric determination of mercury (II) using a glassy carbon electrode modified with gold nanoparticles. Microchim Acta 183:2411–2419. https://doi.org/10.1007/s00604-016-1884-6
Carioni VM, Brockman JD, Morris MC, Ngwenyama RA, Schell LA, Spate VL, Crane S (2018) Instrumental neutron activation analysis, a technique for measurement of Se, Hg, Fe, Zn, K, Mn, Br, and the Hg: Se ratio in brain tissue samples with results from the Memory and Aging Project (MAP). J Radioanal Nucl Chem 318:43–48. https://doi.org/10.1007/s10967-018-6020-0
Liu YM, Zhang FP, Jiao BY, Rao JY, Leng G (2017) Automated dispersive liquid-liquid microextraction coupled to high performance liquid chromatography-cold vapour atomic fluorescence spectroscopy for the determination of mercury species in natural water samples. J Chromatogr A 1493:1–9. https://doi.org/10.1016/j.chroma.2017.03.002
Song X, Ye M, Tang X, Wang C (2013) Ionic liquids dispersive liquid–liquid microextraction and HPLC-atomic fluorescence spectrometric determination of mercury species in environmental waters. J Sep Sci 36:414–420. https://doi.org/10.1002/jssc.201200571
Svechkarev D, Dereka B, Doroshenko A (2011) Mercury ions Complexation with a series of heterocyclic derivatives of 3-hydroxychromone: spectral effects and prospects for ultrasensitive Hg2+ probing. J Phys Chem A 115:4223–4230. https://doi.org/10.1021/jp110974n
Bahta M, Ahmed N (2018) Design and synthesis of 1, 4-benzothiazine hydrazide as selective and sensitive colorimetric and turn-on fluorometric sensor for Hg2+ detection in aqueous medium. J Photochem Photobiol A 357:41–48. https://doi.org/10.1016/j.jphotochem.2018.02.022
Venkatesan P, Thirumalivasan N, Wu SP (2017) A Rhodamine-based chemosensor with diphenylselenium for highly selective fluorescence turn-on detection of Hg2+ in vitro and in vivo. RSC Adv 7:21733–21739. https://doi.org/10.1039/C7RA02459B
Ding H, Zheng C, Li B, Liu G, Pu S, Jia D, Zhou Y (2016) A rhodamine-based sensor for Hg 2+ and resultant complex as a fluorescence sensor for I−. RSC Adv 6:80723–80728. https://doi.org/10.1039/C6RA17861H
Beija M, Afonso CA, Martinho JM (2009) Synthesis and applications of rhodamine derivatives as fluorescent probes. Chem Soc Rev 38:2410–2433. https://doi.org/10.1039/B901612K
Kubin RF, Fletcher AN (1982) Fluorescence quantum yields of some rhodamine dyes. J Lumin 27:455–462. https://doi.org/10.1016/0022-2313(82)90045-X
Zheng A, Chen J, Wu G, Wei H, He C, Kai X, Chen Y (2009) Optimization of a sensitive method for the “switch-on” determination of mercury (II) in waters using Rhodamine B capped gold nanoparticles as a fluorescence sensor. Microchim Acta 164:17–27. https://doi.org/10.1007/s00604-008-0023-4
Wanichacheva N, Hanmeng O, Kraithong S, Sukrat K (2014) Dual optical Hg2+ selective sensing through FRET system of fluorescein and rhodamine B fluorophores. J Photochem Photobiol A 278:75–81. https://doi.org/10.1016/j.jphotochem.2014.01.003
Lakowicz JR (2006) Mechanisms and dynamics of fluorescence quenching. In: (eds) Principles of fluorescence spectroscopy. Springer, Boston, pp 331-351
Vaishanav SK, Korram J, Pradhan P, Chandraker K, Nagwanshi R, Ghosh KK, Satnami ML (2017) Green luminescent CdTe quantum dot based fluorescence nano-sensor for sensitive detection of arsenic (III). J Fluoresce 27:781–789. https://doi.org/10.1007/s10895-016-2011-0
Verma VK, Tapadia K, Maharana T, Sharma A (2018) Convenient and ultra-sensitive fluorescence detection of bovine serum albumin by using Rhodamine-6G modified gold nanoparticles in biological samples. Luminescence 33:1408–1414. https://doi.org/10.1002/bio.3563
Wanichacheva N, Setthakarn K, Prapawattanapol N, Hanmeng O, Lee VS, Grudpan K (2012) Rhodamine B-based “turn-on” fluorescent and colorimetric chemosensors for highly sensitive and selective detection of mercury(II) ions. J Lumin 132:35–40. https://doi.org/10.1016/j.jlumin.2011.07.015
Córdoba MH, Garcia IL, Sánchez-Pedreño C (1984) Spectrophotometric determination of mercury with thiocyanate and Rhodamine B. Microchim Acta 84:467–475. https://doi.org/10.1007/BF01197161
Matveichuk YV, Rakhman’ko EM, Yasinetskii VV (2015) Thiocyanate complexes of d metals: study of aqueous solutions by UV, visible, and IR spectrometry. Russ J Inorg Chem 60:100–104. https://doi.org/10.1134/S0036023615010076
Karami C, Taher MA, Shahlaei M (2020) A simple method for determination of mercury (II) ions by PNBS-doped carbon dots as a fluorescent probe. J Mater Sci Mater Electron:1–9. https://doi.org/10.1007/s10854-020-03157-5
Alizadeh K, Parooi R, Hashemi P, Rezaei B, Ganjali MR (2011) A new Schiff's base ligand immobilized agarose membrane optical sensor for selective monitoring of mercury ion. J Hazard Mater 186:1794–1800. https://doi.org/10.1016/j.jhazmat.2010.12.067
Ciucu A (2010) Fast spectrometric method for mercury (II) determination based on glucose-oxidase inhibition. Anal Lett 43:1377–1386. https://doi.org/10.1080/00032710903518781
Li H, Zhang Y, Wang X, Xiong D, Bai Y (2007) Calixarene capped quantum dots as luminescent probes for Hg2+ ions. Mater Lett 61:1474–1477. https://doi.org/10.1016/j.matlet.2006.07.064
Khan H, Ahmed MJ, Bhanger MI (2005) A simple spectrophotometric determination of trace level mercury using 1, 5-diphenylthiocarbazone solubilized in micelle. Anal Sci 21:507–512. https://doi.org/10.2116/analsci.21.507
Acknowledgments
We acknowledge gratefully to National Institute of Technology, Raipur (CG) India for making availability of essential instrument as well as lab facility. We sincerely show our gratitude to University Grants Commission (UGC), New Delhi, India for providing financial endorsement as fellowship (JRF student id-141394).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There is no conflict to declare in our manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, S.K., Tapadia, K. & Sharma, A. Selective Fluorometric Analysis of Hg(II) in Industrial Waste Water Samples. J Fluoresc 30, 1375–1381 (2020). https://doi.org/10.1007/s10895-020-02627-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02627-7