Abstract
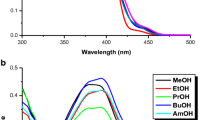
IR-792 as near IR (NIR) laser dye was dissolved with different concentrations in two types of ionic liquids (ILs) of different anion and cation, 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide (EMIM TFSI) & 1-Butyl-3-methylimidazolium tetrafluoroborate (BMIM BF4), as the benign green solvent and in methanol (MeOH) as a standard solvent. The behavior of fluorescence of IR-792 dye was studied. The fluorescence of IR-792 dissolved in the ILs was heavily compared to organic solvent. Some photo-physical parameters of IR-792 were calculated. Mainly, IR-792 had a very low quantum yield of fluorescence with high intersystem crossing rate & fluorescence lifetime in picosecond range. Optical absorption and behavior of fluorescence for the rigorously the purified imidazolium ILs in the neat condition and effect of IR-792 on their fluorescence have been examined. The emission behavior of IR-792 in green solvents was independent upon the wavelength of excitation, while the emission behavior of green solvents dependent upon the wavelength of excitation whether in pure state or with NIR laser dye. At most, the intensity of fluorescence of ILs is dependent upon dye concentration.







Similar content being viewed by others
References
Qiu D, Liu Y, Li M, Chen H, Li H (2017) J Lumin 185:286–291
Dähne S (1978) Science 199:1163–1167
Daehne S, Resch-Genger U, and Wolfbeis OS (1997) Near-infrared dyes for high technology applications. Springer, Dordrecht
E. Terpetschnig and O. S. Wolfbeis (1998) Luminescent probes for NIR sensing applications. In book title: Near-infrared dyes for high technology applications. Springer, Dordrecht, pp 161–182
Patonay G, Salon J, Sowell J, Strekowski L (2004) Noncovalent labeling of biomolecules with red and near- infrared dyes. Molecules 9:40–49
Vinatier V, Guieu V, Madaule Y, Maturano M, Payrastre C, Hoffmann P (2010) Superoxide-induced bleaching of streptocyanine dyes: application to assay the enzymatic activity of superoxide dismutases. Anal Biochem 405:255–259
Venner MRW, Case AD, Fulker DJ (2004) Solid State Lasers XIII: Technol Devices 5332:189–199
Soper SA, Mattingly QL (1994) Steady-state and picosecond laser fluorescence studies of nonradiative pathways in tricarbocyanine dyes: implications to the design of near-IR fluorochromes with high fluorescence efficiencies. J Am Chem Soc 116:3744–3752
Billard I, Moutiers G, Labet A, El Azzi A, Gaillard C, Mariet C, Lützenkirchen K (2003) Stability of divalent europium in an ionic liquid: spectroscopic investigations in 1-methyl-3-butylimidazolium hexafluorophosphate. Inorg Chem 42:1726–1733
Álvaro M, Ferrer B, Garcia H, Narayana M (2002) Chem Phys Lett 362:435–440
Driesen K, Nockemann P, Binnemans K (2004) Ionic liquids as solvents for near-infrared emitting lanthanide complexes. Chem Phys Lett 395:306–310
Somers AE, Howlett PC, MacFarlane DR, Forsyth M (2013) A review of ionic liquid lubricants. Lubricants 1:3–21
Wilkes JS, Zaworotko MJ (1992) J Chem Soc Chem Commun 13:965–967
Pirovano V, Marchetti M, Carbonaro J, Brambilla E, Rossi E, Ronda L, Abbiati G (2020) Synthesis and photophysical properties of isocoumarin-based D-π-A systems. Dyes Pigments 173:107917
Paul A, Mandal PK, Samanta A (2005) How transparent are the imidazolium ionic liquids? A case study with 1-methyl-3-butylimidazolium hexafluorophosphate, [bmim][PF6]. Chem Phys Lett 402:375–379
Hardacre C, Holbrey JD, McMath SEJ, Bowron DT, Soper AK (2003) J Chem Phys 118:273–278
Katayanagi H, Hayashi S, Hamaguchi H, Nishikawa K (2004) Structure of an ionic liquid, 1-n-butyl-3-methylimidazolium iodide, studied by wide-angle X-ray scattering and Raman spectroscopy. Chem Phys Lett 392:460–464
Hu Z, Margulis CJ (2006) Heterogeneity in a room-temperature ionic liquid: persistent local environments and the red-edge effect. Proc Natl Acad Sci 103:831–836
Wang Y, Voth GA (2005) Unique spatial heterogeneity in ionic liquids. J Am Chem Soc 127:12192–12193
Paul A, Mandal PK, Samanta A (2005) On the optical properties of the imidazolium ionic liquids. J Phys Chem B 109:9148–9153
Paul A, Samanta A (2006) Optical absorption and fluorescence studies on imidazolium ionic liquids comprising thebis(trifluoromethanesulphonyl)imide anion. J Chem Sci 118:335–340
AL-Aqmar DM, Abdelkader HI, Kana MTHA (2017) J Mol Liq 231:370–378
Al-Aqmar DM, Abdelkader HI, Kana MTHA (2015) Optical, photo-physical properties and photostability of pyrromethene (PM-597) in ionic liquids as benign green-solvents. J Lumin 161:221–228
Al-Aqmar DM, Abdelkader HI, Kana MTHA (2015) Spectroscopic properties and amplified spontaneous emission of fluorescein laser dye in ionic liquids as green media. Opt Mater 47:573–581
Soriano E, Holder C, Levitz A, Henary M (2016) Molecules 21:23
Kurutos A, Ryzhova O, Tarabara U, Trusova V, Gorbenko G, Gadjev N, Deligeorgiev T (2016) Novel synthetic approach to near-infrared heptamethine cyanine dyes and spectroscopic characterization in presence of biological molecules. J Photochem Photobiol A Chem 328:87–96
Liu X, Atwater M, Wang J, Huo Q (2007) Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf B Biointerfaces 58:3–7
Costela A, Munoz JM, Douhal A, Figuera JM, Acuna AU (1989) Experimental test of a four-level kinetic model for excited-state intramolecular proton transfer dye lasers. Appl Phys B Lasers Opt 49:545–552
J. R. Lakowicz (2013) Principles of fluorescence spectroscopy. Springer Science & Business Media, Springer, Dordrecht
Crosby GA, Demas JN (1971) Measurement of photoluminescence quantum yields. Review. J Phys Chem 75:991–1024
Ruland G, Gvishi R, Prasad PN (1996) Multiphasic nanostructured composite: multi-dye tunable solid state laser. J Am Chem Soc 118:2985–2991
D’Alessio JT, Ludwig PK, Burton M (1964) Ultraviolet lamp for the generation of intense, constant‐shape pulses in the subnanosecond region. Rev Sci Instrum 35:1015–1017
Pavlopoulos TG (2002) Scaling of dye lasers with improved laser dyes. Prog Quantum Electron 26:193–224
Raju B, Varadarajan TS (1995) Photophysical properties and energy transfer dye laser characteristics of 7-diethylamino-3-heteroaryl coumarin in solution. Laser Chem 16:109–120
Sakr MEM, Kana MTHA, Elwahy AHM, El-Daly SA, Ebeid E-ZM (2020) Novel far UV–Vis absorbing bis(dihydrophenanthro[9,10-e][1,2,4]triazine) derivative dyes: synthesis, optical, photophysical and solvatochromic properties. J Mol Struct 1206:127690
Levitz A, Marmarchi F, Henary M (2018) Synthesis and optical properties of near-infrared meso-phenyl-substituted symmetric heptamethine cyanine dyes. Molecules 23:226
Schulman S. G. (2017) Fluorescence and phosphorescence spectroscopy: physicochemical principles and practice. Elsevier
Acknowledgments
The authors would like to thank Cairo University for Supporting and funding this work through project no. (16–33).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
AL-Aqmar, D.M., Al-Shamiri, H.A.S., AL-Shareef, J.M. et al. Spectroscopic and Photo-Physical Properties of Near-IR Laser Dye in Novel Benign Green Solvents. J Fluoresc 30, 1095–1103 (2020). https://doi.org/10.1007/s10895-020-02576-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02576-1