Abstract
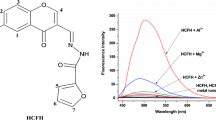
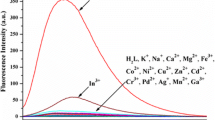
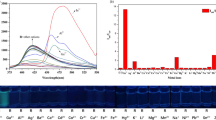
A simple Schiff base (L) based on 2-hydroxyacetophenone and o-phenylenediamine was prepared which acts as an effective fluorescent sensor for Al3+ with ca. 9.0 fold enhancement in fluorescence intensity and detection limit 10–4.3 M. L can quite clearly distinguish Al3+ over other metal ions Zn2+, Hg2+, Cd2+, Pb2+, Mn2+, Mg2+, Co2+, Ni2+, Cu2+, Ca2+, K+, Li+, Na+ and Fe3+. Cyclic voltammogram and square wave voltammogram of L shows a significant change on interaction with Al3+. Spectroscopic data and DFT calculations confirm 1:1 interaction between L and Al3+ which is reversible with respect to Na2EDTA.














Similar content being viewed by others
References
Goswami S, Paul S, Manna A (2013) FRET based ‘red-switch’ for Al 3+ over ESIPT based 'green-switch'for Zn2+: dual channel detection with live-cell imaging on a dyad platform. RSC Adv 3:10639
Verstraeten SV, Aimo L, Oteiza PI (2008) Aluminium and lead: molecular mechanisms of brain toxicity. Arch Toxicol Pharmacol 82:789
Barcelo J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48:75
Altschuler E (1999) Aluminum-containing antacids as a cause of idiopathic Parkinson’s disease. Med Hypotheses 53:22
Wang B, Xing W, Zhao Y, Deng X (2010a) Effects of chronic aluminum exposure on memory through multiple signal transduction pathways. Environ Toxicol Pharmacol 29:308
Walton JR (2006) Aluminum in hippocampal neurons from humans with Alzheimer’s disease. Neuro Toxicol 27:385
Polizzi S, Pira E, Ferrara M, Bugiani M, Papaleo A, Albera R, Palmi S (2002) Neurotoxic effects of aluminium among foundry workers and Alzheimer’s disease. Neuro Toxicol 23:761
Witters HE, Puymbroeck SV, Stouthart AJHX, Bonga SEW (1996) Physicochemical changes of aluminium in mixing zones: Mortality and physiological disturbances in brown trout (Salmo trutta L.). Environ Toxicol Chem 15:986
Wang JL, Zang L, Li YY, You J, Wu P, Zheng SJ (2006) Citrate transporters play a critical role in aluminium-stimulated citrate efflux in rice bean (Vigna umbellata) roots. Ann Bot 97:579
Kochian LV, Hoekenga OA, Pineros MA (2004) How do crop plants tolerate acid soil? Mechanism of aluminium tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107:315
Sang H, Liang P, Du D (2008) Determination of trace aluminum in biological and water samples by cloud point extraction preconcentration and graphite furnace atomic absorption spectrometry detection. J Hazard Mater 154:1127
de Jesus DS, das Graças Korn M, Ferreira SLC, Carvalho MS (2000) A separation method to overcome the interference of aluminium on zinc determination by inductively coupled plasma atomic emission spectroscopy. Spectrochim Acta B 55:389
Abbasi S, Farmany A, Gholivand MB, Naghipour A, Abbasi F, Khani H (2009) Kinetic-spectrophotometry method for determination of ultra trace amounts of aluminum in food samples. Food Chem 116:1019
Gupta VK, Jain AK, Maheswari G (2007) Aluminum(III) selective potentiometric sensor based on morin in poly(vinyl chloride) matrix. Talanta 72:1469
Dutta K, Deka RC, Das DK (2014) The first enhanced ring planarity based fluorescent “off-on” sensor for Ca2+ ion. J Lumin 148:325–329
Das DK, Goswami P, Medhi B (2014) N-benzoate-N′ salicylaldehyde ethynelediamine: a new fluorescent sensor for Zn2+ ion by “off-on” mode. J Fluoresc 145:454–458
Kumar J, Bhattacharyya P, Das DK (2015a) New duel fluorescent “on–off” and colorimetric sensor for Copper(II): Copper(II) binds through N coordination and pi cation interaction to sensor. Spectrochim Acta A 138:99
Gupta VK, Jain AK, Kumawat LK (2014a) Thiazole Schiff base turn-on fluorescent chemosensor for Al3+ ion Sens. Actuators B Chem 195:98
Gupta VK, Singh AK, Mergu N (2014b) Antipyrine based Schiff bases as turn-on fluorescent sensors for Al (III) ion. Electrochim Acta 117:405
Paul S, Manna A, Goswami S (2015) A differentially selective molecular probe for detection of trivalent ions (Al3+, Cr3+ and Fe3+) upon single excitation in mixed aqueous medium. Dalton Trans 44:11805
Kim S, Noh JY, Kim KY, Kim JH, Kang HK, Nam S-W, Kim SH, Park S, Kim C, Kim J (2012) Salicylimine-based fluorescent chemosensor for aluminum ions and application to bioimaging. Inorg Chem 51:3597
Wang L et al (2010b) A simple sensitive ESIPT on-off fluorescent sensor for selective detection of Al3+ in water. Biomol Chem 8:3751
Cramer JC (2004) Essentials of computational chemistry, theories and models, 2nd edn. John Wiley & Sons Ltd, England
Koch WM, Holthausen C (2001) A. Chemist’s Guide to Density Functional Theory, 2nd edn. Wiley-VCH, New York
Fiothais C, Nogueira F, Marques M (eds) (2003) A primer in density functinal theory. Springer-Verlag, Berlin
Cohen AJ, M-Sanchez P, Yang W (2012) Challenges for density functional theory. Chem Rev 112:289
Parr RG, Yang W (1995) Density-functional theory of the electronic structure of molecules. Annu Rev Phys Chem 46:701
Kumar J, Sarma MJ, Phukan P, Das DK (2015b) Surfactant dependent pH controlled “off-on”, “off-on-off” and “on-off” fluorescent switches exhibited by N-benzylidenenaphthalen-1-amine. J Fluoresce 25:1431–1435
Ali SA, Soliman AA, Aboaly MM, Ramadan RM (2002) Chromium, molybdenum and ruthenium complexes of 2-hydroxyacetophenone Schiff bases. J Coord Chem 55(10):1161
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, Revision C.01. Gaussian, Inc., Wallingford CT
Acknowledgements
The authors thank DST, New Delhi for SERB (DST) project No. EMR/2016/001745 and financial support to the Department under FIST.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bharali, B., Talukdar, H., Phukan, P. et al. A New Schiff Base Based Fluorescent Sensor for Al(III) Based on 2-Hydroxyacetophenone and o-Phenylenediamine. J Fluoresc 30, 751–757 (2020). https://doi.org/10.1007/s10895-020-02527-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02527-w