Abstract
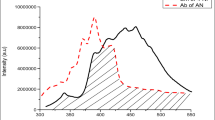
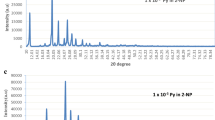
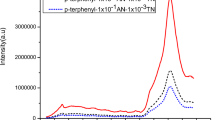
A series of novel luminophors of 2-naphthol by doping anthracene were prepared using conventional solid state reaction technique. The photophysical, electrochemical and thermal properties were studied by Fluorescence spectroscopy, XRD, SEM, TGA-DSC and by Cyclic Voltammetry techniques. The thin films were characterized by Fluorescence spectroscopy. XRD study of fine grained powders exhibited sharp peaks which specify crystallinity and homogeneity of the doped luminophors. The fluorescence spectra of doped 2-naphthol exhibited emission of anthracene at 413 nm i.e. blue emission with instantaneous fluorescence quenching of 2-NP due to excitation energy transfer (EET). Electrochemical data specify that the HOMO and LUMO energy levels of the synthesized luminophors are in the range of 5.55–5.71 eV and 3.03–3.24 eV, respectively. TGA-DSC study confirmed thermal stability of prepared luminophors. Hence, overall study proposes that these luminophors seems applicable to be used as n-type materials for Optoelectronic devices.






Similar content being viewed by others
References
Pron A et al (2011)Triarylamine substituted Arylene Bisimides as solution Processable organic semiconductors for field effect transistors. Effect of substituent position on their spectroscopic, electrochemical, structural, and electrical transport properties. J Phys Chem C 115:15008–15017
Bujak P, Kulszewicz-Bajer I, Zagorska M, Maurel V, Wielgus I, Pron A (2013) Polymers for electronics and spintronics. Chem Soc Rev 42:8895–8999
Lincker F, Heinrich B, De Bettignies R, Rannou P, Pecaut J, GreB B, Donnio RD (2011) Fluorenone core donor–acceptor–donor π-conjugated molecules end-capped with dendritic oligo(thiophene)s: synthesis, liquid crystalline behaviour, and photovoltaic applications. J Mater Chem 21:5238
Friend RH, Gymer RW, Holmes AB, Burroughes JH, Marks RN, Taliani C, Bradley DDC, Santos DAD, Bredas JL, Logdlund M, Salaneck WR (1999) Study on the Optoelectronic Properties of UV Luminescent Polymer: ZnO Nanoparticles Dispersed PANI. Nature 397:121–128
Kim DY, Cho HN, Kim CY (2000) Prog. Synthesis and optical properties of poly[(4-(benzoxazole-2-yl)phenyl)methyl methacrylate] with 1,8-naphthalimide end group. Polym Sci 25:1089
Shinohara H, Kotani M (1980) Singlet energy transfer of p-terphenyl doped anthracene. Bulletin of the Chemical Society of Japan 53:3171
Kohler A, Wilson JS, Friend RH (2002) Fluorescence and phosphorescence in organic materials. Adv Eng Mater 4:453–459
Hung LS, Chen CH (2002) Anthracene derivative for a non-doped blue-emitting organic electroluminescence device with both excellent color purity and high efficiency. Mat Sci Eng R 39:143
Ping Q, Akiyama T, Abe K, Shigenari T (1995) Fluorescence spectra and ifetime of 2-naphthol in H2O- and D2O-ice(Ih) single crystal. Solid State Commun 95(3):177–180
Schab-Balcerzak E et al (2015) New core-substituted with electron-donating group 1,8-naphthalimides towards optoelectronic applications. J Lumin 166:22–39
Wang H, Yang Z, Xie Z, Wang H, Wang B, Ma Y (2014) The thermodynamic characteristics of organic crystal growth by physical vapor transport: towards high-quality and color-tunable crystal preparation. Cryst Eng Comm 16:4539–4545
Ravi G, Srinivasan K, Anbukumar S, Ramasamy P (1994) Growth and characterization of sulphate mixed L-arginine phosphate and ammonium dihydrogen phosphate/potassium dihydrogen phosphate mixed crystals. J Cryst Growth 137:598–604
Desai NK, Kolekar GB, Patil SR (2012) Preparation and characterization of anthracene doped p-terphenyl polycrystalline powders for scintillation application. Int J Lumin Appl 2(I):38–40
Desai NK, Mahajan PG, Kumbhar AS, Kolekar GB, Patil SR (2016) Nanoporous p-terphenyl polystyrene films containing perylene; fabrication, characterization and remarkable fluorescence resonance energy transfer based blue emitting properties. J Mater Sci Mater Electron 27:1118
Desai RR, Lakhminarayana D, Patel PB, Panchal CJ (2002) Electrical and optical properties of indium sesquitelluride (In2Te3) thin films. J Mater Sci 41:2019
Patil SR, Patwari SB (1999) Red shift in fluorescence of naphthalene doped by anthracene and perylene. J Lumin 82:115–119
Desai NK, Gupta MK, Kolekar GB, Patil SR (2013) Fluorescence enhancement effect in pyrene and perylene doped nanoporous polystyrene films: mechanistic and morphology. Phys Status Solidi A 210:2121
Mitsui M, Kawano Y (2013) Electronic energy transfer in tetracene-doped p-terphenyl nanoparticles: extraordinarily high fluorescence enhancement and quenching. Chem Phys 419:30
Masuko M, Ohuchi S, Sode K, Ohtani H, Shimadzu A (2000) Fluorescence resonance energy transfer from pyrene to perylene labels for nucleic acid hybridization assays under homogeneous solution conditions. Nucl Acids Res 28:1
Wang X, Lau KC, Li WK (2011) Doping effects on structural and electronic properties of ladderanes and ladder polysilanes: a density functional theory investigation. J Phys Chem A 115:7656
Pujari SR, Bhosale PN, Rao PMR, Patil SR (2002) Sensitized monomer fluorescence and excitation energy transfer in perylene-doped phenanthrene in crystalline and in polymer matrix. Mater Res Bull 37:439–448
Gharge MN, Bhattar SL, Kolekar GB, Patil SR (2008) Structural and photophysical aspects of perylene- doped anthracene crystalline powders prepared by microwave heating. Indian J Chem Sect A 47:1642–1648
Kalita PK, Sarma BK, Das HL (2000) Structural characterization of vacuum evaporated ZnSe thin films. Bull Mater Sci 23(4):313–317
Marciniak B, Rozycka-Sokolowska E, Pavlyuk V (2003) 2-Naphthalenol. Acta Cryst E59:o52
Shaikh AM, Sharma BK, Kamble RM (2015) Synthesis, Photophysical, electrochemical and thermal studies of Triarylamines based on benzo[g]quinoxalines. J Chem Sci 127(9):1571–1579
Sharma BK, Dixit S, Chacko S, Kamble RM, Agarwal N (2017) Synthesis and studies of Imidazoanthraquinone derivatives for applications in organic electronics. Eur J Org Chem :4389
Acknowledgements
The authors express sincere thanks to IIT Madras, SAIF and Instrumentation Centre, Solapur University, Solapur, Maharashtra-India and D.B.F. Dayanand College of Science, Solapur, Maharashtra -India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mane, K.G., Nagore, P.B. & Pujari, S.R. Synthesis, Photophysical, Electrochemical and Thermal Investigation of Anthracene Doped 2-Naphthol Luminophors and their Thin Films for Optoelectronic Devices. J Fluoresc 28, 1023–1028 (2018). https://doi.org/10.1007/s10895-018-2265-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2265-9