Abstract
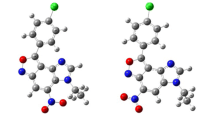
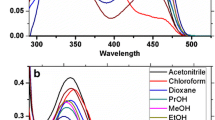
A novel series of pyrazoline derivatives were synthesized and their spectral properties were characterized via FT-IR, 1H, and 13C NMR. The electronic transitions and fluorescence properties were tracked via UV-Vis and emission spectrometry. The density functional theory (DFT) calculations have been also computed to get spot onto the geometry, electronic transitions and spectroscopic properties theoretically that has been compared with the encountered experimental ones. Moreover, the dipole moment, optimized energy, HOMO - LUMO energies and band gaps were calculated for novel candidates pyrazoline derivatives with highly fluorescence quantum yield.














Similar content being viewed by others
References
Lone I, Khan K, Fozdar B (2014) Synthesis, physicochemical properties, antimicrobial and antioxidant studies of pyrazoline derivatives bearing a pyridyl moiety. Med Chem Res 23:363–369
Lv P-C, Li D-D, Li Q-S, Lu X, Xiao Z-P, Zhu H-L (2011) Synthesis, molecular docking and evaluation of thiazolylpyrazoline derivatives as EGFR TK inhibitors and potential anticancer agents. Bioorg Med Chem Lett 21:5374–5377
Babu VH, Sridevi C, Joseph A, Srinivasan KK (2008) Synthesis and biological evaluation of some novel pyrazolines. Indian J Pharm Sci 69:470–473
West RC (ed) (1974) CRC Handbook of Chemistry and Physics, 5th edn. CRC Press, Cleveland
Li JF, Guan B, Li DX, Dong C (2007) Study on the fluorescence properties of a new intramolecular charge transfer compound 1,5- diphenyl-3-(N-ethylcarbazole-3-yl)-2-pyrazoline. Spectrochim Acta A 68:404–408
Hasan A, Abbas A, Akhtar MN (2011) Synthesis, characterization and fluorescent property evaluation of 1, 3, 5-triaryl-2-pyrazolines. Molecules 16:7789–7802
Zimmer H, Armbruster DC, Trauth LJ (1965) The aldol condensation of aromatic aldehydes with N-Acetyl-2-pyrrolidinone: synthesis of 3-Arylidene-2-pyrrolidinones. J Heterocyclic Chem 2:171
Hedaya E, Theodoropulos S (1968) The preparation and reactions of stable phosphorus ylides derived from maleic anhydrides, maleimides or isomaleimides. Tetrahedron 24:2241
Ichikawa M, Masuhara H, Maus M, Rettig W (1996) Radiative depopulation of the excited intramolecular charge-transfer state of 9-(4-(N,N-Dimethylamino)phenyl)phenanthrene. J Am Chem Soc 118:2892
Abbas A, Hussain S, Hafeez N, Naseer MM (2014) Synthesis and spectral characterization of new homologous 1,3,5-triaryl-2-pyrazolines: influence of alkyloxy chain length on fluorescence. Spectrochim Acta A 133:182–189
Lu B, Zhang J, Wang M, Zhou Y, Chen X (2012) Synthesis and fluorescent property of pyrazoline derivatives. Chin J Chem 30:1345–1350
Ozdemir A, Turan-Zitouni G, Kaplancikli ZA et al (2007) Synthesis and antimicrobial activity of 1-(4-aryl-2-thiazolyl)-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives. Eur J Med Chem 42:403–409
Bian B, Ji S-J, Shi H-B (2008) Synthesis and fluorescent property of some novel bischromophore compounds containing pyrazoline and naphthalimide groups. Dyes Pigments 76:348–352
Babar A, Khalid H, Ayub K, Saleem S, Waseem A, Mahmood T, Munawar MA, Abbas G, Khan AF (2014) Synthesis, characterization and density functional theory study of some new 2-anilinothiazoles. J Mol Struct 1072:221–227
Kapturkiewicz A, Herbich J, Karpiuk J, Nowacki J (1997) Intramolecular radiative and radiationless charge recombination processes in donor−acceptor carbazole derivatives. J Phys Chem A 101:2332
Braun D, Retting W, Delmond S, Letard JF, Lapouyade R (1997) Amide derivatives of DMABN: a new class of dual fluorescent compounds. J Phys Chem A 101:6836
Wang P, Wu S (1995) Spectroscopy and photophysics of bridged enone derivatives: effect of molecular structure and solvent. J Photochem Photobiol A Chem 86:109
Wang SL, Ho TI (2000) Substituent effects on intramolecular charge-transfer behaviour of styrylheterocycles. J Photochem Photobiol A Chem 135:119
Hashimoto M, Hamaguchi H (1995) Molecular layer-by-layer engineering of superconducting and superionic materials in the (AgI)Bi2Sr2CaCu2Oy system. J Phys Chem 99:7875
Grabowski ZR (1993) Electron transfer in flexible molecules and molecular ions. Pure Appl Chem 65:1751
Yan ZL, Hu GW, Wu SK (1995) A study on the photophysical 8ehaviors of 1, 5一Diphenyl-3-naphthyl-2-pyrazoline compound. Acta Chim Sin 53:227
Wagner A, Schellhammer CW, Petersen S (1966) Aryl‐Δ2‐pyrazolines as optical brighteners. Angew Chem Int Ed Eng 5:699
Wang P, Komatsuzaki NO, Himeda Y, Sugihara H, Arakawa H, Kasuga K (2001) 3-(2-Pyridyl)-2-pyrazoline derivatives: novel fluorescent probes for Zn2+ion. Tetrahedron Lett 42:9199
Girgis AS, Basta AH, El-Saied H, Mohamed MA, Bedair AH, Salim AS (2018) Synthesis, quantitative structure–property relationship study of novel fluorescence active 2-pyrazolines and application. R Soc Open Sci 5:171964
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc, Wallingford CT
Dennington R, Keith TA, Millam JM (2016) GaussView, Version 6, Semichem Inc., Shawnee Mission, KS
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J ChemPhys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physiol Res B37:785–789
Chinnaraja D, Rajalakshmi R, Srinivasan T, VelmuruganbJ D (2014) Jayabharathi, Spectral studies of 2-pyrazoline derivatives: Structural elucidation through single crystal XRD and DFT calculations. Spectrochim Acta A 124:30–33
Ibrahim MM, Al-Refai M, Ayub K, Ali BF (2016) Synthesis, Spectral Characterization and Fluorescent Assessment of 1,3,5-Triaryl-2-pyrazoline Derivatives: Experimental and Theoretical Studies. J Fluoresc 26:1447–1455
Agrawal M, Sonar PK, Saraf SK (2011) Synthesis of 1,3,5-trisubstituted pyrazoline nucleus containing compounds and screening for antimicrobial activity. Med Chem Res 21:3376–3381
Bhandari S, Tripathi AC, Saraf SK (2013) Novel 2-pyrazoline derivatives as potential anticonvulsant agents. Med Chem Res 22:5290–5296
Acknowledgements
Authors thank anyone who helped us to publish these valuable data presented in our manuscript.
Funding
Authors carried all experiments on their expenses without support from any organization.
Author information
Authors and Affiliations
Contributions
The authors worked jointly on every section of the paper. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Our manuscript didn’t contain any individual person’s data in any form.
Competing Interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Salim, A.S., Girgis, A.S., Basta, A.H. et al. Comparative DFT Computational Studies with Experimental Investigations for Novel Synthesized Fluorescent Pyrazoline Derivatives. J Fluoresc 28, 913–931 (2018). https://doi.org/10.1007/s10895-018-2254-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2254-z