Abstract
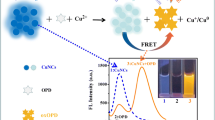
C-phycoerythrin (CPE) was investigated as a colorimetric and fluorometric quantitative sensor for Cu2+ ions in an aqueous medium. UV – visible studies with 50 μM concentration of different metals were carried out with only Cu and Ag showing changes in the absorption spectra. Fluorescence emission studies showed similar results. UV – visible titration of CPE with different [Cu] resulted in a linear relationship within 10 μM Cu and a ‘naked eye’ visible difference in colour, most likely due to the formation of a CPE – Cu complex. Fluorescence emission of CPE was quenched rapidly within 5 min of mixing. Fluorescence emission titration studies revealed gradually decreasing CPE emission with increasing [Cu] with a Stern – Volmer constant of 2.5 × 104 M−1 and a detection limit of 5 μM.. CPE was selective for Cu even in the presence of different metals which were 5 times the concentration of Cu; it was also effective in aqueous samples spiked with Cu. FTIR studies showed considerable changes in the amide III, indicating side chain interactions, even as the protein backbone remained largely unaffected.









Similar content being viewed by others
References
Fawell JK, Ohanion E, Giddings M, et al (2004) Copper in drinking water: Background document for development of WHO Guidelines for Drinking-water Quality. URL: http://www.who.int/water_sanitation_health/dwq/chemicals/copper.pdf. Accessed 16 April 2018
Waggoner DJ, Bartnikas TB, Gitlin JD (1999) The role of copper in neurodegenerative disease. Neurobiol Dis 6:221–230. https://doi.org/10.1006/nbdi.1999.0250
Zeng L, Miller EW, Pralle A et al (2006) A selective turn-on fluorescent sensor for imaging copper in living cells. J Am Chem Soc 128:10–11. https://doi.org/10.1021/ja055064u
Pizarro F, Olivares M, Uauy R et al (1999) Acute gastrointestinal effects of graded levels of copper in drinking water. Environ Health Perspect 107:117–121
Andrew Hong PK, Macauley Y-Y (1998) Corrosion and leaching of copper tubing exposed to chlorinated drinking water. Water Air Soil Pollut 108:457–471. https://doi.org/10.1023/A:1005060206953
Olivares M, Pizarro F, Speisky H et al (1998) Copper in infant nutrition: safety of World Health Organization provisional guideline value for copper content of drinking water. J Pediatr Gastroenterol Nutr 26:251–257
Bond AM, Wallace GG (1981) Determination of copper as a dithiocarbamate complex by reverse-phase liquid chromatography with electrochemical detection. Anal Chem 53:1209–1213. https://doi.org/10.1021/ac00231a018
Monasterios CV, Jones AM, Salin ED (1986) Determination of copper and zinc in human head hair by inductively coupled plasma atomic emission spectrometry with a direct sample insertion device. Anal Chem 58:780–785. https://doi.org/10.1021/ac00295a029
Szpunar J, Bettmer J, Robert M et al (1997) Validation of the determination of copper and zinc in blood plasma and urine by ICP MS with cross-flow and direct injection nebulization. Talanta 44:1389–1396. https://doi.org/10.1016/S0039-9140(97)00009-X
Townsend A, Miller K, McLean S, Aldous S (1998) The determination of copper, zinc, cadmium and lead in urine by high resolution ICP-MS. J Anal At Spectrom 13:1213–1219. https://doi.org/10.1039/A805021J
Tsednee M, Huang Y-C, Chen Y-R, Yeh K-C (2016) Identification of metal species by ESI-MS/MS through release of free metals from the corresponding metal-ligand complexes. Sci Rep 6:26785. https://doi.org/10.1038/srep26785
Lau O-W, Ho S-Y (1993) Simultaneous determination of traces of iron, cobalt, nickel, copper, mercury and lead in water by energy-dispersive x-ray fluorescence spectrometry after preconcentration as their piperazino-1,4-bis(dithiocarbamate) complexes. Anal Chim Acta 280:269–277. https://doi.org/10.1016/0003-2670(93)85131-3
Sauvé S, McBride MB, Hendershot WH (1995) Ion-selective electrode measurements of copper(II) activity in contaminated soils. Arch Environ Contam Toxicol 29:373–379. https://doi.org/10.1007/BF00212503
Camerlynck R, Kiekens L (1982) Speciation of heavy metals in soils based on charge separation. Plant Soil 68:331–339. https://doi.org/10.1007/BF02197938
Cassella RJ, Magalhães OIB, Couto MT et al (2005) Synthesis and application of a functionalized resin for flow injection/F AAS copper determination in waters. Talanta 67:121–128. https://doi.org/10.1016/j.talanta.2005.02.019
Maity D, Manna AK, Karthigeyan D et al (2011) Visible–near-infrared and fluorescent copper sensors based on julolidine conjugates: selective detection and fluorescence imaging in living cells. Chem Eur J 17:11152–11161. https://doi.org/10.1002/chem.201101906
Mariappan K, Alaparthi M, Caple G et al (2014) Selective fluorescence sensing of copper(II) and water via competing imine hydrolysis and alcohol oxidation pathways sensitive to water content in aqueous acetonitrile mixtures. Inorg Chem 53:2953–2962. https://doi.org/10.1021/ic402723c
Park GJ, Hwang IH, Song EJ et al (2014) A colorimetric and fluorescent sensor for sequential detection of copper ion and cyanide. Tetrahedron 70:2822–2828. https://doi.org/10.1016/j.tet.2014.02.055
Wang M, Leung K-H, Lin S et al (2014) A colorimetric chemosensor for Cu2+ ion detection based on an iridium(III) complex. Sci Rep 4:6794. https://doi.org/10.1038/srep06794
Jena BK, Raj CR (2008) Gold nanoelectrode ensembles for the simultaneous electrochemical detection of ultratrace arsenic, mercury, and copper. Anal Chem 80:4836–4844. https://doi.org/10.1021/ac071064w
Annadhasan M, Muthukumarasamyvel T, Sankar Babu VR, Rajendiran N (2014) Green synthesized silver and gold nanoparticles for colorimetric detection of Hg2+, Pb2+, and Mn2+ in aqueous medium. ACS Sustain Chem Eng 2:887–896. https://doi.org/10.1021/sc400500z
Chen H, Zhang J, Liu X et al (2014) Colorimetric copper(ii) ion sensor based on the conformational change of peptide immobilized onto the surface of gold nanoparticles. Anal Methods 6:2580–2585. https://doi.org/10.1039/C3AY42211A
Jeevika A, Ravi Shankaran D (2014) Visual colorimetric sensing of copper ions based on reproducible gelatin functionalized silver nanoparticles and gelatin hydrogels. Colloids Surf A Physicochem Eng Asp 461:240–247. https://doi.org/10.1016/j.colsurfa.2014.08.002
Ye Y, Guo Y, Yue Y et al (2015) Colorimetric sensing of copper ions based on the anti-aggregation of unmodified silver nanoparticles in the presence of 1,4-dithiothreitol. Anal Methods 7:566–572. https://doi.org/10.1039/C4AY02359E
Zhao Q, Yan H, Liu P et al (2016) An ultra-sensitive and colorimetric sensor for copper and iron based on glutathione-functionalized gold nanoclusters. Anal Chim Acta 948:73–79. https://doi.org/10.1016/j.aca.2016.10.024
Ghosh T, Bhayani K, Paliwal C et al (2016) Cyanobacterial pigments as natural anti-hyperglycemic agents: an in vitro study. Front Mar Sci 3:146. https://doi.org/10.3389/fmars.2016.00146
Mishra SK, Shrivastav A, Mishra S (2011) Preparation of highly purified C-phycoerythrin from marine cyanobacterium Pseudanabaena sp. Protein Expr Purif 80:234–238. https://doi.org/10.1016/j.pep.2011.06.016
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435. https://doi.org/10.1083/jcb.58.2.419
Lakowicz JR (2006) Quenching of fluorescence. In: Lacowicz (ed) Principles of Fluorescence Spectroscopy, 3 ed. Springer US, Boston, MA, pp 277–330. https://doi.org/10.1007/978-0-387-46312-4_8
O’Fagain C (2004) Storage of Pure Proteins. In: Cutler P (ed) Protein purification protocols, 2 ed. Humana Press, Totowa, NJ, pp 323–337. https://doi.org/10.1385/1-59259-655-X:323
Barth A (2007) Infrared spectroscopy of proteins. Biochim Biophys Acta (BBA) - Bioenerg 1767:1073–1101. https://doi.org/10.1016/j.bbabio.2007.06.004
Acknowledgements
The manuscript has been assigned registration number CSIR-CSMCRI – 031/2018. TG would like to thank the Academy of Scientific and Innovative Research for Ph. D. registration and the Council of Scientific and Industrial Research for financial support through its projects (OLP 0040, CSC 0105). AV would like to thank Devi Ahilya Vishwavidyalaya, Indore and CSIR-Central Salt and Marine Chemicals Research Institute for the opportunity to conduct her dissertation. KB would like to thank the Council of Scientific and Industrial Research for financial support (CSC 0203) and Maharaja Krishnakumarsinhji Bhavnagar University for Ph. D. registration. All the authors are highly grateful to AESDCIF, CSIR-CSMCRI, Bhavnagar for providing the instrumentation facilities and analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, T., Vyas, A., Bhayani, K. et al. C-Phycoerythrin as a Colorimetric and Fluorometric Probe for the Sensitive, Selective and Quantitative Detection of Cu2+ in Aqueous Samples. J Fluoresc 28, 671–680 (2018). https://doi.org/10.1007/s10895-018-2229-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2229-0