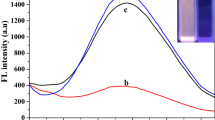
Abstract
Experimental studies in the aqueous solution are crucial for the detection of metal cations in environmental and biological media. Polymer materials allow to work in aqueous media for fluorescent substances which are insoluble in water. Fluorescence sensor studies of the phenanthroimidazole imine compound (PI) synthesized previously by our group were performed in the polymer medium and the selective response to Fe (III) cation was obtained. The resulting sensor exhibited a selective fluorescence quenching effect to Fe (III). A logarithmic calibration graph was obtained in the range of 5.0 × 10− 5 and 1.0 × 10− 2 M. The interference effects of other ions besides Fe (III) have been examined and it has been observed that PI compound behaves selectively to Fe (III) ion in aqueous media. In addition, regeneration and reproducibility studies were carried out to determine the long-term usage of PI doped polymer film and reproducible results have been obtained for Fe (III) cation.








Similar content being viewed by others
References
The Editors of Encyclopædia (2017) Britannica, https://global.britannica.com/science/iron-chemical-element
Rouault TA (2006) The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol 2:406–414
Gupta VK, Jain AK, Agarwal S, Maheshwari G (2007) An iron(III) ion-selective sensor based on a µ-bis(tridentate) ligand. Talanta 71:1964–1968
Qin W, Zhang ZJ, Wang FC (1998) Chemiluminescence flow system for the determination of Fe(II) and Fe(III) in water. J Anal Chem 360:130–132
Lin W, Long L, Yuan L, Cao Z, Feng J (2009) A novel ratiometric fluorescent Fe3+ sensor based on a phenanthroimidazole chromophore. Anal Chim Acta 634:262–266
Ohashi A, Ito H, Kanai C, Imura H, Ohashi K (2005) Cloud point extraction of iron(III) and vanadium(V) using 8-quinolinol derivatives and Triton X-100 and determination of 10(-7)moldm(-3) level iron(III) in riverine water reference by a graphite furnace atomic absorption spectroscopy. Talanta 65:525–530
Pomazal K, Prohaska C, Steffan I, Reich G, Huber JFK (1999) Determination of Cu, Fe, Mn, and Zn in blood fractions by SEC-HPLC-ICP-AES coupling. Analyst 124:657–663
Ugo P, Moretto LM, Boni AD, Scopece P, Mazzocchin GA (2002) Iron(II) and iron(III) determination by potentiometry and ion-exchange voltammetry at ionomer-coated electrodes. Anal Chim Acta 474:147–160
Bakkaus E, Collins RN, Morel JL, Gouget B (2006) Anion exchange liquid chromatography–inductively coupled plasma-mass spectrometry detection of the Co2+, Cu2+, Fe3 + and Ni2 + complexes of mugineic and deoxymugineic acid. J Chromatogr A 1129:208–215
Xu J-H, Hou Y-M, Mab Q-J, Wub X-F, Wei X-J (2013) Highly selective fluorescent sensor for Fe3 + based on covalently immobilized derivative of naphthalimide. Spectrochim Acta Part A Mol Biomol Spectrosc 112:116–124
Hua CJ, Zheng H, Zhang K, Xin M, Gao J, Li Y (2016) A novel turn off fluorescent sensor for Fe(III) and pH environment based on coumarin derivatives: the fluorescence characteristics and theoretical study. Tetrahedron 72:8365–8372
Valeur B, Leray I (2005) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205:3–40
Nie S, Zare RN (1997) Optical detection of single molecules. Annu Rev 26:567–596
Hu W, Boateng D, Kong J, Zhang X (2015) Advancement of fluorescent methods for detection of Nitric Oxide. Austion J Biosens Bioelectron 1:1003
Azmi SNH, Al-Hadhrami SSK, Al-Marhoubi BMR, Al-Sulaimi SSS, Al-Shamoosi ZDS (2017) Development and validation of fluorescence spectrophotometric method: quantitation of chlorpheniramine maleate in pharmaceutical formulations. J Mol Liq 243:750–760
Kuzu B, Tan M, Ekmekci Z, Menges N (2017) A novel fluorescent sensor based on imidazole derivative for Fe3+ ions. J Lumin 192:1096–1103
Li X, Yin Y, Deng J, Zhong H, Thang J, Chen Z, Yang L, Ma LJ (2016) A solvent-dependent fluorescent detection method for Fe3+ and Hg2+ based on a rhodamine B derivative. Talanta 154:329–334
Zhao B, Liu T, Fang Y, Wang L, Song B, Deng Q (2016) Two ‘Turn-off’ Schiff base fluorescence sensors based on phenanthro[9,10-d]imidazole-coumarin derivatives for Fe3+ in aqueous solution. Tetrahedron Lett 57:4417–4423
Oter O, Ertekin K, Kılıncarslan R, Ulusoy M, Cetinkaya B (2007) Photocharacterization of a novel fluorescent Schiff base and investigation of its utility as an optical Fe3+ sensor in PVC matrix. Dyes Pigm 74:730–735
Tofiño PP, Moreno JM, Pérez-Conde MC (2000) A flow-through fluorescent sensor to determine Fe(III) and total inorganic iron. Talanta 51:537–545
Gulle S, Celik Erbas S, Uzel A, Synthesis and Spectroscopic Studies of Phenanthroimidazole Imine Derivatives and Evaluation of Their Antioxidant Activity. J. Fluoresc https://doi.org/10.1007/s10895-017-2184-1
Gispert JR (2008) Coordination Chemistry,Wiley-VCH. 483. ISBN 352731802X
Klein HF, Camadanli S, Beck R, Leukel D, Flörke U (2005) Cyclometalation of Substrates Containing Imine and Pyridyl Anchoring Groups by Iron and Cobalt Complexes. Angew Chem Int Ed 44: 975 – 977
Pearson RG (1963) Hard and Soft Acids and Bases. J Am Chem Soc 85:3533–3539
Acknowledgements
Funding for this research was provided by the project 2013-096, Scientific Research Funds of Manisa Celal Bayar University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gülle, S., Çelik Erbaş, S. A Selective Fluorescence Sensor for Fe (III) Based on Phenanthroimidazole Imine Compound. J Fluoresc 28, 445–451 (2018). https://doi.org/10.1007/s10895-017-2207-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2207-y