Abstract
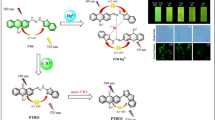
An efficient and highly selective pyrene-thiophene conjugate has been reported as a dual sensor for Hg2+ and cysteamine (an important drug for genetic disorder). The sensor displays a turn-on fluorescence response towards Hg2+ in a 2:1 stoichiometric ratio via excimer formation with a detection limit as low as of 30.6 nM. The excimer emission upon binding with Hg2+ has been rationalized by experimental as well as theoritical studies. Moreover, the [probe-Hg2+] adduct functions as an efficient sensor for cysteamine. This sensing process happens via the extraction of Hg2+ from the adduct. In this paper, change in emission properties of the receptor with varying pH and water content has also been explained. The sensing abilities of the sensor were examined in real water sample analysis. Therefore, the sensor can be used as an efficient and reusable fluorescent sensor for recognition of Hg2+ in water.











Similar content being viewed by others
References
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environmental Science Technology 47:4967–4983. https://doi.org/10.1021/es305071v
Mudgal V, Madaan N, Mudgal A, Singh RB, Mishra S (2010) Effect of Toxic Metals on Human Health. Open Nutraceuticals J 3:94–99. https://doi.org/10.1007/s00244-010-0120-9 doi
Kim HN, Ren WX, Kim JS, Yoon J (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium and mercury ions. Chem Soc Rev 41:3210–3244. https://doi.org/10.1039/C1CS15245A
Fitzgerald WF, Lamborg CH, Hammerschmidt CR (2007) Marine Biogeochemical Cycling of Mercury. Chem Rev 107:641–662. https://doi.org/10.1021/cr050353m
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36:609–662. https://doi.org/10.1080/10408440600845619
Mahato P, Saha S, Das P, Agarwal H, Das A (2014) An overview of the recent developments on Hg2+ recognition. RSC Adv 4:36140–36174. https://doi.org/10.1039/C4RA03594A
Wang Q, Kim D, Dionysiou DD, Sorial GA, Timberlake D (2004) Sources and remediation for mercury contamination in aquatic systems–a literature review. Environ Pollut 131:323–336. https://doi.org/10.1016/j.envpol.2004.01.010
Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18:149–175. https://doi.org/10.1002/tox.10116
Mercury Update: Impact on Fish Advisories; EPA Fact Sheet EPA- 823-F-01–001; Environmental Protection Agency., Office of Water: Washington, DC, 2001
Lisha KP, Anshup, Pradeep T (2009) Towards a practical solution for removing inorganic mercury from drinking water using gold nanoparticles. Gold Bull 42:144–152
Anthemidis AN, Zachariadis GA, Kougoulis JS, Stratis JA (2002) Flame atomic absorption spectrometric determination of chromium(VI) by on-line preconcentration system using a PTFE packed column. Talanta 57:15–22. https://doi.org/10.1016/S0039-9140(01)00676-2
Moreno F, Garcia-Barrera T, Gomez-Ariza JL (2010) Simultaneous analysis of mercury and selenium species including chiral forms of selenomethioninein human urine and serum by HPLC column-switching coupled to ICP-MS. Analyst 135:2700–2705. https://doi.org/10.1039/C0AN00090F
Yu JC, Lo JM, Wai KM (1983) Extraction of gold and mercury from sea water with bismuth diethyldithiocarbamate prior to neutron activation- γ-spectrometry. Anal Chim Acta 154:307–312. https://doi.org/10.1016/0003-2670(83)80032-4
Bennun L, Gomez J (1997) Determination of mercury by total-reflection X-ray fluorescence using amalgamation with gold. Spectrochim Acta Part B 52:1195–1200. https://doi.org/10.1016/S0584-8547(97)00003-7
Safawi A, Eddon L, Foulkes M, Stockwell P, Corns W (1999) Determination of total mercury in hydrocarbons and natural gas condensate by atomic fluorescence spectrometry. Analyst 124:185–189. https://doi.org/10.1039/A809679A
Zejli H, Sharrock P, de Cisneros JLHH., Naranjo-Rodriguez I, Temsamani KR (2005) Voltammetric determination of trace mercury at a sonogel–carbon electrode modified with poly-3-methylthiophene. Talanta 68:79–85. https://doi.org/10.1016/j.talanta.2005.04.060
Liu Z, He W, Guo Z (2013) Metal coordination in photoluminescent sensing. Chem Soc Rev 42:1568–1600. https://doi.org/10.1039/C2CS35363F
Sikdar A, Roy S, Haldar K, Sarkar S, Panja SS (2013) Rhodamine-Based Cu2+ -Selective Fluorosensor: Synthesis, Mechanism, and Application in Living Cells. J Fluoresc 23:495–501. https://doi.org/10.1007/s10895-013-1169-y
Chiou YR, Wan CF, Wu AT (2017) A Selective Colorimetric and Turn-on Fluorescent Chemosensor for Hg2+ in Aqueous Solution. J Fluoresc 27:317–322. https://doi.org/10.1007/s10895-016-1960-7
Yu SY, Wu SP (2014) A highly selective turn-on fluorescence chemosensor for Hg(II) and its application in living cell imaging. Sensors Actuators B Chem 201:25–30. https://doi.org/10.1016/j.snb.2014.04.077
Mei Q, Tian R, Shi Y, Hua Q, Chen C, Tong B (2016) A series of selective and sensitive fluorescent sensors based on a thiophen-2-yl-benzothiazole unit for Hg2+. New J Chem 40:2333–2342. https://doi.org/10.1039/C5NJ02259B
Ding H, Zheng C, Li B, Liu G, Pu S, Jia D, Zhou Y (2016) A rhodamine-based sensor for Hg2+ and resultant complex as a fluorescence sensor for I–. RSC Adv 6:80723–80728. https://doi.org/10.1039/C6RA17861H
Chang IJ, Hwang KS, Chang SK (2017) Selective Hg2+ signaling via dithiane to aldehyde conversion of an ESIPT fluorophore. Dyes Pigm 137:69–74. https://doi.org/10.1016/j.dyepig.2016.09.058
Jun ME, Roy B, Ahn KH (2011) “Turn-on” fluorescent sensing with “reactive” probes. Chem Commun 47:7583 – 7601. https://doi.org/10.1039/c1cc00014d
Wang F, Nandhakumar R, Moon JH, Kim KM, Lee JY, Yoon J (2011) Ratiometric Fluorescent Chemosensor for Silver Ion at Physiological pH. Inorg Chem 50:2240–2245. https://doi.org/10.1021/ic1018967
Sarkar S, Roy S, Sikdar A, Saha RN, Panja SS (2013) A pyrene-based simple but highly selective fluorescence sensor for Cu2+ ions via a static excimer mechanism. Analyst 138:7119–7126. https://doi.org/10.1039/C3AN00928A
Banerjee A, Karak D, Sahana A, Guha S, Lohar S, Das D (2011) Methionine–pyrene hybrid based fluorescent probe for trace level detection and estimation of Hg(II) in aqueous environmental samples: Experimental and computational studies. J Hazard Mater 186:738–744. https://doi.org/10.1016/j.jhazmat.2010.11.060
Yang Y, Gou X, Blecha J, Cao H (2010) A highly selective pyrene based fluorescent sensor toward Hg2+ detection. Tetrahedron Lett 51:422–425. https://doi.org/10.1016/j.tetlet.2010.04.100
Sivaraman G, Anand T, Chellappa D (2012) Development of a pyrene based ‘‘turn on’’ fluorescent chemosensor for Hg2+. RSC Adv 2:10605–10609. https://doi.org/10.1039/C2RA21202A
Shellaiah M, Rajan YC, Balu P, Murugan A (2015) A Pyrene based schiff base probe for selective fluorescent turn-on detection of Hg2+ ions with live cell application. New J Chem 39:2523–2531. https://doi.org/10.1039/C4NJ02367F
Suresh M, Mishra S, Mishra SK, Suresh E, Mandal AK, Shrivastav A, Das (2009) A Resonance energy Transfer Approach and a New Ratiometric Probe for Hg2+ in Aqueous Media and Living Organism. Org Lett 11:2740–2743. https://doi.org/10.1021/ol900810q
Zhou Y, You XY, Fang Y, Li JY, Liu K, Yao C (2010) A thiophen-thiooxorhodamine conjugate fluorescent probe for detecting mercury in aqueous media and living cells. Org Biomol Chem 8:4819–4822. https://doi.org/10.1039/C0OB00452A
Mandal S, Banerjee A, Lohar S, Chattopadhyay A, Sarkar B, Mukhopadhyay SK, Sahana A, Das D (2013) Selective sensing of Hg2+ using rhodamine–thiophene conjugate: Red light emission and visual detection of intracellular Hg2+ at nanomolar level. Journal of Hazardous Materials 261:198–205. https://doi.org/10.1016/j.jhazmat.2013.07.026
Adhikari S, Ghosh A, Mandal S, Sengupta A, Chattopadhyay A, Matalobos JS, Lohar S, Das D (2014) Visible light excitable ON fluorescence and naked eye detection of Cu2+ via hydrolysis of rhodamine–thiophene conjugate: human breast cancer cell (MCF7) imaging studies. Dalton Trans 43:7747–7751. https://doi.org/10.1039/c4dt00002a
Tang W, Xiang Y, Tong A (2009) Salicylaldehyde Azines as Fluorophores of Aggregation-Induced Emission Enhancement Characteristics. J Org Chem 74:2163–2166. https://doi.org/10.1021/jo802631m
Benesi HA, Hildebrand JH (1949) A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J Am Chem Soc 71:2703–2707. https://doi.org/10.1021/ja01176a030
Das S, Sahana A, Banerjee A, Lohar S, Safin DA, Babashkina MG, Bolte M, Garcia Y, Hauli I, Mukhopadhyay SK, Das D (2013) Ratiometric fluorescence sensing and intracellular imaging of Al3+ ions driven by an intramolecular excimer formation of a pyrimidine–pyrene scaffold. Dalton Trans 42:4757–4763. https://doi.org/10.1039/C3DT32908A
Shiraishi Y, Ishizumi K, Nishimura G, Hirai T (2007) Effects of Metal Cation Coordination on Fluorescence Properties of a Diethylenetriamine Bearing Two End Pyrene Fragments. J Phys Chem B 111:8812–8822. https://doi.org/10.1021/jp072081s
Yang RH, Chan WH, Lee AWM, Xia PF, Zhang HK, Li K (2003) A Ratiometric Fluorescent Sensor for AgI with High Selectivity and Sensitivity. J Am Chem Soc 125:2884–2885. https://doi.org/10.1021/ja029253d
Hong Y, Lam JWY, Tang BZ (2011) Aggregation-induced emission. Chem Soc Rev 40:5361–5388. https://doi.org/10.1039/C1CS15113D
Shellaiah M, Simon T, Srinivasadesikan V, Lin CM, Sun KW, Ko FH, Lin MC, Lin HC (2016) Novel pyrene containing monomeric and dimeric supramolecular AIEE active nano-probes utilized in selective “off–on” trivalent metal and highly acidic pH sensing with live cell applications. J Mater Chem C 4:2056–2071. https://doi.org/10.1039/C5TC03738G
Lakowicz JR (2006) Principles of Fluorescence Spectroscopy, 3rd edn. Springer, New York
Gaussian, 03, Rev.C.02, Gaussian Inc. (2004) Wallingford CT
Acknowledgements
Authors thankfully acknowledge DST-FIST and NIT Durgapur for creating and providing infrastructural facility for research. SS also acknowledge CSIR (01(2904)/17/EMR-II) for financial support. The authors are thankful to The University of Burdwan for ESI-mass spectra and IIT Kharagpur for 1HNMR spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sarkar, S., Roy, S., Saha, R.N. et al. Thiophene Appended Dual Fluorescent Sensor for Detection of Hg2+ and Cysteamine. J Fluoresc 28, 427–437 (2018). https://doi.org/10.1007/s10895-017-2204-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2204-1