Abstract
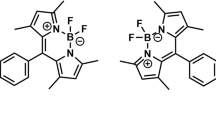
Photophysical properties of BODIPY dyes containing acetyl acetone and benzoyl acetone BF2 unit as an electron accepting substituent at beta position linked via double bond have been investigated using a wide range of solvents of different polarities. The substitution effect at beta position of the BODIPY dyes on their absorption, emission and quantum yield of fluorescence have been the aim of present study. For the synthesized BODIPY dyes fluorescence quantum yields and lifetimes show very sharp decrease with an increase in the solvent polarity, suggesting the involvement of highly polar ICT state de-excitation mechanism along with the local excitation process. The polarity dependent changes in average fluorescence life time and quantum yield values rationalize the formation of ICT states.










Similar content being viewed by others
References
Chibani S, Charaf-Eddin A, Le Guennic B, Jacquemin D (2013) Boranil and related NBO dyes: insights from theory. J Chem Theory Comput 9:3127–3135. https://doi.org/10.1021/ct400392r
Zhang Z, Achilefu S (2005) Design, synthesis and evaluation of near-infrared fluorescent pH indicators in a physiologically relevant range. Chem Commun 5887–5889. https://doi.org/10.1039/B512315A
Boens N, Leen V, Dehaen W (2012) Fluorescent indicators based on BODIPY. Chem Soc Rev 41:1130–1172. https://doi.org/10.1039/C1CS15132K
Prandi C, Ghigo G, Occhiato EG, Scarpi D, Begliomini S, Lace B, Alberto G, Artuso E, Blangetti M (2014) Tailoring fluorescent strigolactones for in vivo investigations: a computational and experimental study. Org Biomol Chem 12:2960–2968. https://doi.org/10.1039/c3ob42592d
Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K (2013) BODIPY dyes in photodynamic therapy. Chem Soc Rev 42:77–88. https://doi.org/10.1039/C2CS35216H
Gong S, Liu Q, Wang X, Xia B, Liu Z, He W (2015) AIE-active organoboron complexes with highly efficient solid-state luminescence and their application as gas sensitive materials. Dalton Trans 44:14063–14070. https://doi.org/10.1039/C5DT01525A
Mukherjee S, Thilagar P (2016) Stimuli and shape responsive “boron-containing” luminescent organic materials. J Mater Chem C 4:2647–2662. https://doi.org/10.1039/C5TC02406D
Loudet A, Burgess K (2007) Dyes BODIPY and their derivatives: syntheses and spectroscopic properties. Chem Rev 107:4891–4932. https://doi.org/10.1021/cr078381n
Zhu S, Bi J, Vegesna G, Zhang J, Luo F-T, Valenzano L, Liu H (2013) Functionalization of BODIPY dyes at 2,6-positions through formyl groups. RSC Adv 3:4793–4800. https://doi.org/10.1039/C3RA22610G
Buyukcakir O, Bozdemir OA, Kolemen S, Erbas S, Akkaya EU (2009) Tetrastyryl-bodipy dyes: convenient synthesis and characterization of elusive near IR fluorophores. Org Lett 11:4644–4647. https://doi.org/10.1021/ol9019056
Mani V, Krishnakumar VG, Gupta S, Mori S, Gupta I (2017) Synthesis and characterization of styryl-BODIPY derivatives for monitoring in vitro Tau aggregation. Sensors Actuators B Chem 244:673–683. https://doi.org/10.1016/j.snb.2016.12.104
Zhao N, Vicente MGH, Fronczek FR, Smith KM (2015) Synthesis of 3,8-dichloro-6-ethyl-1,2,5,7-tetramethyl-BODIPY from an asymmetric dipyrroketone and reactivity studies at the 3,5,8-positions. Chemistry 21 6181–6192. https://doi.org/10.1002/chem.201406550
Lakshmi V, Ravikanth M (2014) Synthesis of hexasubstituted boron-dipyrromethenes having a different combination of substituents. Eur J Org Chem 2014 5757–5766. https://doi.org/10.1002/ejoc.201402599
Rohand T, Baruah M, Qin W, Boens N, Dehaen W (2006) Functionalisation of fluorescent BODIPY dyes by nucleophilic substitution. Chem Commun 266–268. https://doi.org/10.1039/B512756D
Dost Z, Atilgan S, Akkaya EU (2006) Distyryl-boradiazaindacenes: facile synthesis of novel near IR emitting fluorophores. Tetrahedron 62:8484–8488. https://doi.org/10.1016/j.tet.2006.06.082
Kim E, Felouat A, Zaborova E, Ribierre J-C, Wu JW, Senatore S, Matthews C, Lenne P-F, Baffert C, Karapetyan A, Giorgi M, Jacquemin D, Ponce-Vargas M, Le Guennic B, Fages F, D’Aleo A (2016) Borondifluoride complexes of hemicurcuminoids as bio-inspired push-pull dyes for bioimaging. Org Biomol Chem 14:1311–1324. https://doi.org/10.1039/C5OB02295A
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JV, Ortiz J, Cioslowski, Fox DJ (2009) Gaussian 09, Revision C.01, Gaussian 09, Revis. B.01, Gaussian, Inc., Wallingford CT. citeulike-article-id:9096580
Bauernschmitt R, Häser M, Treutler O, Ahlrichs R (1997) Calculation of excitation energies within time-dependent density functional theory using auxiliary basis set expansions. Chem Phys Lett 264:573–578. https://doi.org/10.1016/S0009-2614(96)01343-7
Becke AD (1993) Density functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Yue Y, Guo Y, Xu J, Shao S (2011) A Bodipy-based derivative for selective fluorescence sensing of homocysteine and cysteine. New J Chem 35:61–64. https://doi.org/10.1039/C0NJ00720J
Jiao L, Yu C, Li J, Wang Z, Wu M, Hao E (2009) β-formyl-BODIPYs from the Vilsmeier-Haack reaction. J Org Chem 74:7525–7528. https://doi.org/10.1021/jo901407h
Liu K, Chen J, Chojnacki J, Zhang S (2013) BF3·OEt2-promoted concise synthesis of difluoroboron-derivatized curcumins from aldehydes and 2,4-pentanedione. Tetrahedron Lett 54:2070–2073. https://doi.org/10.1016/j.tetlet.2013.02.015
More AB, Mula S, Thakare S, Chakraborty S, Ray AK, Sekar N, Chattopadhyay S (2017) An acac-BODIPY dye as a reversible “ON-OFF-ON” fluorescent sensor for Cu2 + and S2- ions based on displacement approach. J Lumin 190:476–484. https://doi.org/10.1016/j.jlumin.2017.06.005
Satpati AK, Kumbhakar M, Nath S, Pal H (2008) Photoinduced electron transfer between quinones and amines in micellar media: tuning the Marcus inversion region. J Photochem Photobiol A Chem 200:270–276. https://doi.org/10.1016/j.jphotochem.2008.08.001
Shaikh M, Pal H (2014) Photophysics of donor-acceptor kind of styryl dyes: involvement of twisted intramolecular charge transfer (TICT) state and the effect of solvent polarity
Saroja G, Soujanya T, Ramachandram B, Samanta A (1998) 4-Aminophthalimide derivatives as environment-sensitive probes. J Fluoresc 8:405–410. https://doi.org/10.1023/A:1020536918438
Pham THN, Clarke RJ (2008) Solvent dependence of the photochemistry of the styrylpyridinium dye RH421. J Phys Chem B 112:6513–6520. https://doi.org/10.1021/jp711694u
Popere BC, Della Pelle AM, Thayumanavan S (2011) BODIPY-based donor-acceptor pi-conjugated alternating copolymers. Macromolecules 44:4767–4776. https://doi.org/10.1021/ma200839q
More AB, Mula S, Thakare S, Sekar N, Ray AK, Chattopadhyay S (2014) Masking and Demasking Strategies for the BF2-BODIPYs as a Tool for BODIPY Fluorophores. J Org Chem 79:10981–10987. https://doi.org/10.1021/jo502028g
Acknowledgements
ABM sincerely thanks DAE-BRNS for the financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
More, A.B., Chakraborty, G., Mula, S. et al. Modulation of the Photophysical Properties of β-substituted BODIPY Dyes. J Fluoresc 28, 381–392 (2018). https://doi.org/10.1007/s10895-017-2200-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2200-5