Abstract
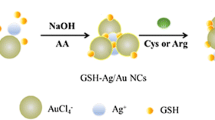
In this report, a novel one-step chemical reduction method was reported for synthesis of water-soluble and stable fluorescent glutathione-templated silver nanocluster (GSH-Ag NCs) with ascorbic acid as an environmental-friendly reducing agent. On the basis of an oxidoreduction-induced fluorescence quenching mechanism, the prepared GSH-Ag NCs found to act as a cheap, non-toxic and highly sensitive “turn-off” fluorophore for ascorbic acid (AA). Furthermore, the fluorescence of the fluorophore/AA system could be recovered through addition of arginine (Arg), which made the system function as a highly selective “turn-on” sensor for arginine. Therefore, a “turn-off-on” switch sensor was proposed for detection of AA and Arg. Under optimized conditions, the probe gives a fluorescent response that is linear in the 2–300 μM concentration range of AA, with a detection limit of 0.1 μM. The probe for Arg, in turn, has a linear range in the 10–180 μM concentration range, and the limit of detection is 0.5 μM. In addition, the developed method showed great accuracy when employed to detect AA and Arg in human urine and serum, which shows its great potential in biological molecular recognition applications.








Similar content being viewed by others
References
O’Connell PJ, Gormally C, Pravda M, Guilbault GG (2001) Development of an amper-ometric L-ascorbic acid (vitamin C) sensor based on electropolymerised anilinefor pharmaceutical and food analysis. Anal Chim Acta 431:239–247
Zhang L, Cai H, Zhou C et al (2015) Facile and fast fabrication of polyaniline nanorods on acidized titanium foils with a synergistic effect for electrochemical sensing. J Mater Chem B 3(44):8629–8637
Johnston CS, Steinberg FM, Rucker RB et al (2001) Ascorbic acid
Gopalakrishnan V, Burton PJ, Blaschke TF (1996) High-performance liquid chromatographic assay for the quantitation of L-arginine in human plasma. Anal Chem 68:3520–3523
Wu GY, Morris SM, Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Vishwanathan K, Tackett RL, Stewart JT et al (2000) Determination of arginine and methylated arginines in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B 748:157–166
Stechmiller JK, Childress B, Cowan L (2005) Arginine supplementation and wound healing. Nutr Clin Pract 20:52–61
Zhou X, Jin X, Li D et al (2011) Selective detection of zwitterionic arginine with a new Zn (II)-terpyridine complex: potential application in protein labeling and determination. Chem Commun 47:3921–3923
Li J, Zhu JJ, Xu K (2014) Fluorescent metal nanoclusters: from synthesis to applications. Tr Anal Chem 58:90–98
Shiang YC, Huang CC, Chen WY et al (2012) Fluorescent gold and silver nanoclusters for the analysis of biopolymers and cell imaging. J Mater Chem 22:12972–12982
Risse T, Shaikhutdinov S, Nilius N et al (2008) Gold supported on thin oxide films: from single atoms to nanoparticles. Accounts Chem Res 41:949–956
Shang L, Dong S, Nienhaus GU (2011) Ultra-small fluorescent metal nanoclusters: synthesis and biological applications. Nano Today 6:401–418
Zheng J, Zhang C, Dickson RM (2004) Highly fluorescent, water-soluble, size-tunable gold quantum dots. Phys Rev Lett 93:077402
Yan Z, Hui FQ, Anindita DAS et al (2011) Comparison of the catalytic properties of 25 atom gold nanospheres and nanorods. Chinese J Catal 32:1149–1155
Yu Y, Luo Z, Chevrier DM et al (2014) Identification of a highly luminescent Au22(SG)18 nanocluster. J Am Chem Soc 136:1246–1249
Yuan X, Setyawati MI, Xie JP et al (2013) Highly luminescent silver nanoclusters with tunable emissions: cyclic reduction–decomposition synthesis and antimicrobial properties. NPG Asia Mater 5:e39
Huang S, Pfeiffer C, Hollmann J, Friede S, Chen JJ (2012) Synthesis and characterization of colloidal fluorescent silver nanoclusters. Langmuir 28:8915–8919
Templeton AC, Wuelfing WP, Murray RW (2000) Monolayer-protected cluster molecules. Acc Chem Res 33:27–36
Templeton AC, Chen S, SMG et al (1998) Water-soluble, isolable gold clusters protected by tiopronin and coenzyme a monolayers. Langmuir 15:66–76
Wuelfing WP, Zamborini FP, Templeton AC et al (2000) Monolayer-protected clusters: molecular precursors to metal films. Chem Mater 13:87–95
Negishi Y, Takasugi Y, Sato S et al (2004) Magic-numbered Au(n) clusters protected by glutathione monolayers (n = 18, 21, 25, 28, 32, 39): isolation and spectroscopic characterization. J American Chem Soc 126:6518–6529
Negishi Y, Nobusada K, Tsukuda T (2005) Glutathione-protected gold clusters revisited: bridging the gap between gold(I)-thiolate complexes and thiolate-protected gold nanocrystals. J American Chem Soc 127:5261–5270
Jin R (2010) Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2:343–362
Mathew A, Sajanlal PR, Pradeep T (2011) A fifteen atom silver cluster confined in bovine serum albumin. J Mater Chem 21:11205–11212
Zheng KY, Yuan X, Xie JP (2014) Recent advances in synthesis, characterization, and biomedical application of ultrasmall thiolated silver nanoclusters. RSC Adv 4:60581–60596
Liu T, Su Y, Song H et al (2013) Microwave-assisted green synthesis of ultrasmall fluorescent water-soluble silver nanoclusters and its application in chiral recognition of amino acids. Analyst 138:6558–6564
Xu HX, Suslick KS (2010) Sonochemical synthesis of highly fluorescent Ag nanoclusters. ACS Nano 4:3209–3214
Zhang N, Qu F, Luo HQ et al (2013) Sensitive and selective detection of biothiols based on target-induced agglomeration of silvernanoclusters. Biosens and Bioelectron 42:214–218
Yuan X, Luo Z, Zhang Q et al (2011) Synthesis of highly fluorescent metal (Ag, Au, Pt, and Cu) nanoclusters by electrostatically induced reversible phase transfer. ACS Nano 5:8800–8808
Yang XH, Ling J, Peng J et al (2013) Catalytic formation of silver nanoparticles by bovine serum albumin protected-silver nanoclusters and its application for colorimetric detection of ascorbic acid. Spectrochim Acta A 106:224–230
Pandey I, Jha SS (2015) Molecularly imprinted polyaniline-ferrocene-sulfonic acid-carbon dots modified pencil graphite electrodes for chiral selective sensing of D-ascorbic acid and L-ascorbic acid: a clinical biomarker for preeclampsia. Electrochim Acta 182:917–928
Wu T, Guan Y, Ye J (2007) Determination of flavonoids and ascorbic acid in grapefruit peel and juice by capillary electrophoresis with electrochemical detection. Food Chem 100:1573–1579
Chen H, Li W, Zhao P et al (2015) A CdTe/CdS quantum dots amplified graphene quantum dots anodic electrochemiluminescence platform and the application for ascorbic acid detection in fruits. Electrochim Acta 178:407–413
Abdelwahab AA, Shim YB (2015) Simultaneous determination of ascorbic acid, dopamine, uric acid and folic acid based on activated graphene/MWCNT nanocomposite loaded Au nanoclusters. Sensor Actuat B-Chem 221:659–665
Peng J, Ling J, Zhang XQ et al (2015) A rapid, sensitive and selective colorimetric method for detection of ascorbic acid. Sensor Actuat B-Chem 221:708–716
Ferreira DCM, Giordano GF, Mendes RK et al (2015) Optical paper-based sensor for ascorbic acid quantification using silver nanoparticles. Talanta 141:188–194
Cen Y, Tang J, Kong XJ et al (2015) A cobalt oxyhydroxide-modified upconversion nanosystem for sensitive fluorescence sensing of ascorbic acid in human plasma. Nanoscale 7:13951–13957
Liu JJ, Chen ZT, Tang DS et al (2015) Graphene quantum dots-based fluorescent probe for turn-on sensing of ascorbic acid. Sensor Actuat B-Chem 212:214–219
Stechmiller JK, Childress B, Cowan L (2015) Arginine supplementation and wound healing. Nutr Clin Pract 20:52–61
Wang X, Wu P, Hou X, et al (2013) An ascorbic acid sensor based on protein-modified Au nanoclusters. Analyst 138:229–233
Hu L, Deng L, Alsaiari S et al (2014) “light-on” sensing of antioxidants using gold nanoclusters. Analyt Chem 86:4989–4994
Cao J, Ding L, Hu W et al (2014) Ternary system based on fluorophore-surfactant assemblies-Cu2+ for highly sensitive and selective detection of arginine in aqueous solution. Langmuir 30:15364–15372
He L, So VLL, Xin JH (2014) A new rhodamine-thiourea/Al 3+, complex sensor for the fast visual detection of arginine in aqueous media. Sensor Actuat B-Chem 192:496–502
Zhou X, Jin X, Li D et al (2011) Selective detection of zwitterionic arginine with a new Zn(II)-terpyridine complex: potential application in protein labeling and determination. Chem Commun 47:3921–3929
Wei YK, Yang J (2007) Evanescent wave infrared chemical sensor possessing a sulfonated sensing phase for the selective detection of arginine in biological fluids. Talanta 71:2007–2014
Ren HB, Yan XP (2012) Ultrasonic assisted synthesis of adenosine triphosphate capped manganese-doped ZnS quantum dots for selective room temperature phosphorescence detection of arginine and methylated arginine in urine based on supramolecular Mg2+-adenosine triphosphate. Talanta 97:16–22
Pu W, Zhao H, Huang C et al (2013) Visual detection of arginine based on the unique guanidino group-induced aggregation of gold nanoparticles. Anal Chim Acta 764:78–83
Wehner M, Schrader T, Finocchiaro P et al (2000) A chiral sensor for arginine and lysine. Org Lett 2:605–608
Hassen WM, Martelet C, Davis F et al (2006) Calix[4] arene based molecules for amino-acid detection. Sensor Actuat B-Chem 124:38–45
Kenneth S, Hugo S, Peter S (1987) Förster transfer calculations based on crystal structure data from Agmenellum quadruplicatum C-phycocyanin. Photochem Photobiol 46:427–440
Lakowicz JR, Weber G (1973) Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry 12:4171–4179
Jones RM, Bergstedt TS, McBranch DW (2001) Tuning of superquenching in layered and mixed fluorescent polyelectrolytes. J American Chem Soc 123:6726–6727
Murphy CB, Zhang Y, Troxler T (2004) Probing Förster and Dexter energy-transfer mechanisms in fluorescent conjugated polymer chemosensors. J Phys Chem B 108:1537–1543
Baptista MS, Indig GL (1998) Effect of BSA binding on photophysical and photochemical properties of triarylmethane dyes. J Phys Chem B 102:4678–4688
Lakowicz JR (1983) Introduction to fluorescence. Springer, US
Acknowledgments
This work was financially supported by the Natural Science Foundation of Hubei Province (2015CFB273, 2011CDB059 and 2011CDA111).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, N., He, Y., Ge, Y. et al. “Turn-Off-On” Fluorescence Switching of Ascorbic Acid-Reductive Silver Nanoclusters: a Sensor for Ascorbic Acid and Arginine in Biological Fluids. J Fluoresc 27, 293–302 (2017). https://doi.org/10.1007/s10895-016-1957-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1957-2