Abstract
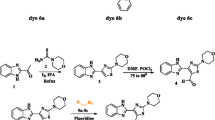
Donor-π- acceptor styryl chromophores based on indole core were synthesized by Knoevenagel condensation of N-ethyl indole-3-carbaldehyde and different active methylene compounds. The absorption and emission properties of these dyes in different solvents were studied. The dyes displayed a broad absorption maximum in the UV and visible region between 397 and 469 nm with FWHM, 50 to 75 nm. Due to the extended π – conjugated systems this styryl chromophores shows strong intramolecular charge transfer characteristics. The dyes showed solid state emission and emission in solid state was red shifted as compared to their emission in less polar solvents. Density Functional Theory [B3LYP/6–311 + G(d)] computations were used to correlate the structural, molecular, electronic and photo physical parameters of styryl dye with experimental study. Synthesized dyes were confirmed by using FT-IR, 1H NMR, 13C NMR and HRMS spectral analysis.








Similar content being viewed by others
References
Matsui M, Ito A, Kotani M, et al. (2009) Dyes and pigments the use of indoline dyes in a zinc oxide dye-sensitized solar cell. Dyes Pigments 80:233–238. doi:10.1016/j.dyepig.2008.07.010
Yu X, Ci Z, Liu T, et al. (2014) Influence of different electron acceptors in organic sensitizers on the performance of dye-sensitized solar cells. Dyes Pigments 102:126–132. doi:10.1016/j.dyepig.2013.10.045
Pommeret S, Gustavsson T, Naskrecki R, et al. (1995) Femtosecond absorption and emission spectroscopy of the DCM laser dye. J Mol Liq 64:101–112. doi:10.1016/0167-7322(95)92824-U
Shettigar S, Umesh G, Poornesh P, et al. (2009) Dyes and pigments the third-order nonlinear optical properties of novel styryl dyes. Dyes Pigments 83:207–210. doi:10.1016/j.dyepig.2009.04.009
Huang JY, Lewis a, Loew L (1988) Nonlinear optical properties of potential sensitive styryl dyes. Biophys J 53:665–670. doi:10.1016/S0006–3495(88)83147–3
Jha BN, Banerji JC (1985) Chromophoric chain β-aryl-substituted styryl cyanines: effect of substituents on visible absorption spectra and photosensitisation properties. Dyes Pigments 6:213–226. doi:10.1016/0143-7208(85)80018-8
Hagberg DP, Yum JH, Lee H, et al. (2008) Novel near-infrared cyanine dyes for fluorescence imaging in biological systems. J Am Chem Soc 130:6259–6266. doi:10.1021/ja800066y
Li Q, Lin G-L, Peng B-X, Li Z-X (1998) Synthesis, characterization and photographic properties of some new styryl cyanine dyes. Dyes Pigments 38:211–218. doi:10.1016/S0143–7208(97)00088–0
Lee CC, Hu AT (2003) Synthesis and optical recording properties of some novel styryl dyes for DVD-R. Dyes Pigments 59:63–69. doi:10.1016/S0143-7208(03)00098-6
Yashchuk VM, Yarmoluk SM, Kudrya VY, et al. (2008) Optical Biomedical Diagnostics: Sensors with Optical Response Based on Two-Photon Excited Luminescent Dyes for Biomolecules Detection. Adv Opt Technol 2008:1–11. doi:10.1155/2008/908246
Park KH, Lee CJ, Song D, et al. (2001) Optical recording properties of styryl derivatives for digital versatile disc-recordable (DVD-R). Mol Cryst Liq Cryst Sci Technol Sect A Mol Cryst Liq Cryst 370:165–168. doi:10.1080/10587250108030062
Sokołowska J, Podsiadły R, Stoczkiewicz J (2008) Styryl dyes as new photoinitiators for free radical polymerization. Dyes Pigments 77:510–514. doi:10.1016/j.dyepig.2006.11.011
Haidekker M, Ling T, Anglo M, et al. (2001) New fluorescent probes for the measurement of cell membrane viscosity. Chem Biol 8:123–131. doi:10.1016/S1074-5521(00)90061-9
Metsov S, Simov D, Stoyanov S, Nikolov P (1990) Photophysical characteristics of some 2-styrylindolium dyes. Dyes Pigments 13:11–19. doi:10.1016/0143-7208(90)80009-E
Kim SD, Park EJ (2001) Relation between chemical structure of yellow disperse dyes and their lightfastness. Fibers Polym 2:159–163. doi:10.1007/BF02875330
Chung S-J, Zheng S, Odani T, et al. (2006) Extended squaraine dyes with large two-photon absorption cross-sections. J Am Chem Soc 128:14444–14445. doi:10.1021/ja065556m
Lee H-S, Kim S-K, Lee W-J, et al. (2001) Synthesis and properties of organic light-emitting diodes using new emissive materials. Mol Cryst Liq Cryst Sci Technol Sect A Mol Cryst Liq Cryst 370:39–42. doi:10.1080/10587250108030034
Fei X, Gu Y, Li C, et al. (2013) Synthesis and spectra of a kind of novel longer-wavelength benzoxazole indole styryl cyanine dye with a carbazole-bridged chain. J Fluoresc 23:221–226. doi:10.1007/s10895-012-1137-y
Babür B, Seferoğlu N, Aktan E, et al. (2014) Phenylazoindole dyes 2: the molecular structure characterizations of new phenylazo indoles derived from 1,2-dimethylindole. Dyes Pigments 103:62–70. doi:10.1016/j.dyepig.2013.11.019
Greaves A, Daubresse N (2009) Thiol/disulfide indole-derived, styryl dye, dye composition comprising said dye, for lightening keratin materials using this dye. WO2009037325 A2
Application F, Data P (2010) ( 12 ) United States Patent. 2:
Mitschke U, Bäuerle P (2000) The electroluminescence of organic materials. J Mater Chem 10:1471–1507. doi:10.1039/a908713c
Chen C-T (2004) Evolution of red organic light-emitting diodes: materials and devices. Chem Mater 16:4389–4400. doi:10.1021/cm049679m
D’Andrade BW, Forrest SR (2004) White organic light-emitting devices for solid-state lighting. Adv Mater 16:1585–1595. doi:10.1002/adma.200400684
Song Y, Di C, Yang X, et al. (2006) A cyclic triphenylamine dimer for organic field-effect transistors with high performance. J Am Chem Soc 128:15940–15941. doi:10.1021/ja064726s
De S, Das S, Girigoswami A (2005) Environmental effects on the aggregation of some xanthene dyes used in lasers. Spectrochim Acta A Mol Biomol Spectrosc 61:1821–1833. doi:10.1016/j.saa.2004.06.054
Liao L, Dai L, Smith A, et al. (2007) Photovoltaic-active Dithienosilole-containing polymers. Macromolecules 40:9406–9412. doi:10.1021/ma071825x
Asefa A, Singh AK (2009) A fluorescence study of novel styrylindoles in homogenous and microhetrogeneous media. J Fluoresc 19:921–930. doi:10.1007/s10895-009-0525-4
Asefa A, Singh AK (2010) Fluorescence emission enhancement in substituted 3-styrylindoles in the solid state. J Lumin 130:24–28. doi:10.1016/j.jlumin.2009.07.016
McDonagh C, Burke CS, MacCraith BD (2008) Optical chemical sensors. Chem Rev 108:400–422. doi:10.1021/cr068102g
Li Q, Lee JS, Ha C, et al. (2004) Solid-phase synthesis of styryl dyes and their application as amyloid sensors. Angew Chem Int Ed 43:6331–6335. doi:10.1002/anie.200461600
Hong Y, Lam JWY, Tang BZ (2011) Aggregation-induced emission. Chem Soc Rev 40:5361. doi:10.1039/c1cs15113d
Wu Q, Peng Q, Niu Y, et al. (2012) Theoretical insights into the aggregation-induced emission by hydrogen bonding: a QM/MM study. J Phys Chem A 116:3881–3888. doi:10.1021/jp3002367
Wang B, Qian Y (2013) Synthesis and efficient solid-state emission of conjugated donor–acceptor–donor triphenylamine chromophores. New J Chem 37:1402. doi:10.1039/c3nj41115j
Rao KV, Datta KKR, Eswaramoorthy M, George SJ (2013) Highly pure solid-state white-light emission from solution-processable soft-hybrids. Adv Mater 25:1713–1718. doi:10.1002/adma.201204407
Matsui M, Ooiwa K, Okada A, et al. (2013) Dyes and pigments solid-state fl uorescence of pyridinium styryl dyes. Dyes Pigments 99:916–923. doi:10.1016/j.dyepig.2013.07.026
Gupta VD, Tathe AB, Padalkar VS, et al. (2013) Red emitting solid state fluorescent triphenylamine dyes: synthesis, photo-physical property and DFT study. Dyes Pigments 97:429–439. doi:10.1016/j.dyepig.2012.12.024
Wang Q, Liu H, Lu H, et al. (2013) Synthesis, characterization and solid-state emission properties of arylsilyl-substituted pyrene derivatives. Dyes Pigments 99:771–778. doi:10.1016/j.dyepig.2013.07.003
Frisch MJ, Trucks GW, Schlegel HB, et al. (2009) Gaussian 09, Revision C.01. Gaussian 09, Revis. B.01, Gaussian, Inc., Wallingford CT
Cancès E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032. doi:10.1063/1.474659
Treutler O, Ahlrichs R (1995) Efficient molecular numerical integration schemes. J Chem Phys 102:346. doi:10.1063/1.469408
Becke AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98:1372. doi:10.1063/1.464304
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Meech SR, Phillips D (1983) Photophysics of some common fluorescence standards. J Photochem 23:193–217. doi:10.1016/0047-2670(83)80061-6
Saroj MK, Sharma N, Rastogi RC (2011) Solvent effect profiles of absorbance and fluorescence spectra of some indole based chalcones. J Fluoresc 21:2213–2227. doi:10.1007/s10895-011-0926-z
Xu H, Fan L-L (2011) Antifungal agents. Part 4: synthesis and antifungal activities of novel indole[1,2-c]-1,2,4-benzotriazine derivatives against phytopathogenic fungi in vitro. Eur J Med Chem 46:364–369. doi:10.1016/j.ejmech.2010.10.022
Rajagopal B, Chou CH, Chung CC, Lin PC (2014) Synthesis of substituted 3-indolylimines and indole-3-carboxaldehydes by rhodium(II)-catalyzed annulation. Org Lett 16:3752–3755. doi:10.1021/ol501618z
Ghanadzadeh Gilani A, Moghadam M, Zakerhamidi MS (2012) Solvatochromism of Nile red in anisotropic media. Dyes Pigments 92:1052–1057. doi:10.1016/j.dyepig.2011.07.018
Hermant RM, Bakker NAC, Scherer T, et al. (1990) Systematic study of a series of highly fluorescent rod-shaped donor-acceptor systems. J Am Chem Soc 112:1214–1221. doi:10.1021/ja00159a050
Ravi M, Samanta A, Radhakrishnan TP (1994) Excited state dipole moments from an efficient analysis of Solvatochromic stokes shift data. J Phys Chem 98:9133–9136. doi:10.1021/j100088a007
Lippert E (1957) Spektroskopische Bestimmung des Dipolmomentes aromatischer Verbindungen im ersten angeregten Singulettzustand. Zeitschrift für Elektrochemie, Berichte der Bunsengesellschaft für Phys Chemie 61:962–975. doi:10.1002/bbpc.19570610819
Mataga N (1963) Solvent effects on the absorption and fluorescence spectra of Naphthylamines and isomeric Aminobenzoic acids. Bull Chem Soc Jpn 36:654–662. doi:10.1246/bcsj.36.654
Mataga N, Kaifu Y, Koizumi M (1955) The solvent effect on fluorescence spectrum, change of solute-solvent interaction during the lifetime of excited solute molecule. Bull Chem Soc Jpn 28:690–691. doi:10.1246/bcsj.28.690
Mataga N, Kaifu Y, Koizumi M (1956) Solvent effects upon fluorescence spectra and the dipolemoments of excited molecules. Bull Chem Soc Jpn 29:465–470. doi:10.1246/bcsj.29.465
Christian Reichardt Solvents and Solvent Effects in Organic Chemistry. Third, Upd:Wiley VCH Verlag GmbH & Co. KGaA, Weinheim.
Reichardt C (1994) Solvatochromic dyes as solvent polarity indicators. Chem Rev 94:2319–2358. doi:10.1021/cr00032a005
Ravi M, Soujanya T, Samanta A, Radhakrishnan TP (1995) Excited-state dipole moments of some Coumarin dyes from a solvatochromic method using the solvent polarity parameter, E N T. J Chem Soc Faraday Trans 91:2739. doi:10.1039/ft9959102739
Kumar S, Rao V, Rastogi R (2001) Excited-state dipole moments of some hydroxycoumarin dyes using an efficient solvatochromic method based on the solvent polarity parameter, ETN. Spectrochim Acta Part A Mol Biomol Spectrosc 57:41–47. doi:10.1016/S1386–1425(00)00330–9
Masternak A, Wenska G, Milecki J, et al. (2005) Solvatochromism of a novel betaine dye derived from purine. J Phys Chem A 109:759–766. doi:10.1021/jp047098e
Nemkovich NA, Pivovarenko VG, Baumann W, et al. (2005) Dipole moments of 4′-aminoflavonol fluorescent probes in different solvents. J Fluoresc 15:29–36. doi:10.1007/s10895-005-0210-1
Aaron JJ, Maafi M, Kersebet C, et al. (1996) A solvatochromic study of new benzo[a]phenothiazines for the determination of dipole moments and specific solute-solvent interactions in the first excited singlet state. J Photochem Photobiol A Chem 101:127–136. doi:10.1016/S1010-6030(96)04442-5
Harbison GS (2002) The electric dipole polarity of the ground and low-lying metastable excited states of NF. J Am Chem Soc 124:366–367. doi:10.1021/ja0159261
Raikar US, Renuka CG, Nadaf YF, et al. (2006) Rotational diffusion and solvatochromic correlation of coumarin 6 laser dye. J Fluoresc 16:847–854. doi:10.1007/s10895-006-0126-4
Nadaf YF, Mulimani BG, Gopal M, Inamdar SR (2004) Ground and excited state dipole moments of some exalite UV laser dyes from solvatochromic method using solvent polarity parameters. J Mol Struct THEOCHEM 678:177–181. doi:10.1016/j.theochem.2004.01.049
Józefowicz M, Heldt JR (2007) Dipole moments studies of fluorenone and 4-hydroxyfluorenone. Spectrochim Acta Part A Mol Biomol Spectrosc 67:316–320. doi:10.1016/j.saa.2006.07.019
Biradar DS, Siddlingeshwar B, Hanagodimath SM (2008) Estimation of ground and excited state dipole moments of some laser dyes. J Mol Struct 875:108–112. doi:10.1016/j.molstruc.2007.04.005
Turro NJ (1978) Modern Molecular Photochemistry. Benjamin-Cummings, New York
Lanke SK, Sekar N (2015) Rigid Coumarins: a Complete DFT, TD-DFT and Non Linear Optical Property Study. J Fluoresc. doi:10.1007/s10895–015–1638-6
Telore RD, Satam M a, Sekar N (2015) NLOphoric studies in thiazole containing symmetrical push – pull fluorophores – Combined experimental and DFT approach. Opt Mater (Amst) 48:271–280. doi:10.1016/j.optmat.2015.08.006
Coe BJ, Harris JA, Asselberghs I, et al. (2002) Quadratic nonlinear optical properties ofN-aryl Stilbazolium dyes. Adv Funct Mater 12:110–116. doi:10.1002/1616–3028(20020201)12:2<110::AID-ADFM110>3.0.CO;2-Y
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular-orbital methods. IX An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724–728. doi:10.1063/1.1674902
Hehre WJ, Ditchfield R, Pople JA (1972) Self—consistent molecular orbital methods. XII. Further extensions of Gaussian—type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257–2261. doi:10.1063/1.1677527
Lanke SK, Sekar N (2016) Aggregation induced emissive carbazole-based push pull NLOphores: synthesis, photophysical properties and DFT studies. Dyes Pigments 124:82–92. doi:10.1016/j.dyepig.2015.09.013
Kleinman DA (1962) Nonlinear dielectric polarization in optical media. Phys Rev 126:1977–1979. doi:10.1103/PhysRev.126.1977
Vidya S, Ravikumar C, Hubert Joe I, et al. (2011) Vibrational spectra and structural studies of nonlinear optical crystal ammonium D, L-tartrate: a density functional theoretical approach. J Raman Spectrosc 42:676–684. doi:10.1002/jrs.2743
Telore RD, Satam M a, Sekar N (2015) Push–pull fluorophores with viscosity dependent and aggregation induced emissions insensitive to polarity. Dyes Pigments 122:359–367. doi:10.1016/j.dyepig.2015.07.017
Oudar JL (1977) Optical nonlinearities of conjugated molecules. Stilbene derivatives and highly polar aromatic compounds. J Chem Phys 67:446. doi:10.1063/1.434888
De PR (1982) Nonlinear-Optical Properties. Phys Rev A 26:2016–1027
Kwon OP, Jazbinsek M, Seo JI, et al. (2010) First hyperpolarizability orientation in asymmetric pyrrole-based polyene chromophores. Dyes Pigments 85:162–170. doi:10.1016/j.dyepig.2009.10.019
Weaver CS, Smith SW, Hyndman RD, et al. (1991) 0.028. Science 252:103–106
Cheng L-T, Tam W, Stevenson SH, et al. (1991) Experimental investigations of organic molecular nonlinear optical polarizabilities. 1. Methods and results on benzene and stilbene derivatives. J Phys Chem 95:10631–10643. doi:10.1021/j100179a026
Dirk CW, Cheng L-T, Kuzyk MG (1992) A simplified three-level model describing the molecular third-order nonlinear optical susceptibility. Int J Quantum Chem 43:27–36. doi:10.1002/qua.560430106
Kuzyk MG, Dirk CW (1990) Effects of centrosymmetry on the nonresonant electronic third-order nonlinear optical susceptibility. Phys Rev A 41:5098–5109. doi:10.1103/PhysRevA.41.5098
Acknowledgments
Santosh Chemate (JRF/SRF) is thankful for fellowship from the Principal Scientific Adviser (PSA), Government of India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 1104 kb)
Rights and permissions
About this article
Cite this article
Chemate, S., Sekar, N. Indole-Based NLOphoric Donor-π-Acceptor Styryl Dyes: Synthesis, Spectral Properties and Computational Studies. J Fluoresc 26, 2063–2077 (2016). https://doi.org/10.1007/s10895-016-1901-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1901-5