Abstract
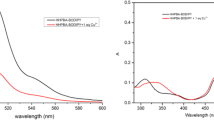
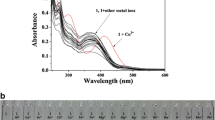
A new fluorescent chemosensor (Bodipy-S) derived from Bodipy and Salophen was developed. After the characterization of all compounds, the behavior of the chemosensor Bodipy-S toward p, d and f block-metal ions was investigated by UV-vis and fluorescence spectroscopy. This chemosensor can selectively detect to Cu (II) in methanol-aqueous solution based on chelation enhanced fluorescence (CHEF) and it almost exhibit to a fluorescence quenching effect with 20-fold. The binding constant of the fluorophore was interpreted by using of the Stern-Volmer method and the complex stoichiometry was defined by using Job’s plot. Moreover, the effect of pH was performed by the fluorescence intensities of Bodipy-S in presence of Cu(II) ions. The chemosensor can be successfully used to the detection of Cu(II) in most areas.









Similar content being viewed by others
References
Gündüz ZY, Gündüz C, Ozpınar C, Urucu OA (2014) A novel Schiff-base as a Cu(II) ion fluorescent sensor in aqueous solution. Spectrochim Acta A Mol Biomol Spectrosc 136:1679–1683
Zhang JR, Zeng AL, Luo HQ, Li NB (2016) Fluorescent silver nanoclusters for ultrasensitive determination of chromium(VI) in aqueous solution. J Hazard Mater 304:66–72
Kacmaz S, Ertekin K, Mercan D, Oter O, Cetinkaya E, Celik E (2015) An ultra sensitive fluorescent nanosensor for detection of ionic copper. Spectrochim Acta A Mol Biomol Spectrosc 135:551–559
Alici O (2016) A novel tripodal colorimetric and fluorescence “turn on” chemosensor for AcO− and F− anions in CH3CN. Spectrochim Acta A Mol Biomol Spectrosc 167:78–83
Banerjee A, Karak D, Sahana A, Guha S, Lohar S, Da D (2011) Methionine–pyrene hybrid based fluorescent probe for trace level detection and estimation of Hg(II) in aqueous environmental samples: Experimental and computational studies. J Hazard Mater 186:738–744
Kagit R, Yildirim M, Ozay O, Yesilot S, Ozay H (2014) Phosphazene based Multicentered naked-eye fluorescent sensor with high selectivity for Fe3+ ions. Inorg Chem 53(4):2144–2151
Malkondu S, Erdemir S (2015) A novel perylene-bisimide dye as “turn on” fluorescent sensor for Hg2+ ion found in DMF/H2O. Dyes Pigments 113:763–769
Brewer GJ (2012) Metals in the causation and treatment of Wilson’s disease and Alzheimer’s disease, and copper lowering therapy in medicine. Inorg Chim Acta 393:135–141
Zetzsche A, Schunter N, Zentek J, Pieper R (2016) Accumulation of copper in the kidney of pigs fed high dietary zinc is due to metallothionein expression with minor effects on genes involved in copper metabolism. J Trace Elem Med Biol 35:1–6
Meng L, Yang S, Feng M, Qu R, Li Y, Liu J, Wang Z, Sun C (2016) Toxicity and bioaccumulation of copper in Limnodrilus hoffmeisteri under different pH values: Impacts of perfluorooctane sulfonate. J Hazard Mater 305:219–228
Rocha GS, Lombardi AT, Melão MGG (2016) Influence of phosphorus on copper toxicity to Selenastrum gracile (Reinsch) Korshikov. Ecotoxicol Environ Saf 128:30–35
Sowada N, Stiller B, Kubisch C (2016) Increased copper toxicity in Saccharomyces cerevisiae lacking VPS35, a component of the retromer and monogenic Parkinson disease gene in humans. Biochem Biophys Res Commun 476:528–533
Thomas G, Andresen E, Mattusch J, Hubáček T, Küpper H (2016) Deficiency and toxicity of nanomolar copper in low irradiance—a physiological and metalloproteomic study in the aquatic plant Ceratophyllum demersum. Aquat Toxicol 177:226–236
Lahman SE, Trent KR, Moore PA (2015) Sublethal copper toxicity impairs chemical orientation in the crayfish, Orconectes rusticus. Ecotoxicol Environ Saf 113:369–377
Çiftçi GY, Şenkuytu E, Durmuş M, Yuksel F, Kılıç A (2013) Fluorenylidene bridged cyclotriphosphazenes: ‘turn-off’ fluorescence probe for Cu2+ and Fe3+ ions. Dalton Trans 42:14916–14926
Huang J, Liu M, Ma X, Dong Q, Ye B, Wang W, Zeng W (2014) A highly selective turn-off fluorescent probe for Cu(II) based on a dansyl derivative and its application in living cell imaging. RSC Adv 4:22964–22970
Xu W-J, Qi D-Q, You J-Z, Hu F-F, Bian J-Y, Yang C-X, Huang J (2015) Coumarin-based ‘turn-off’ fluorescent chemosensor with high selectivity for Cu2+ in aqueous solution. J Mol Struct 1091:133–137
Obali AY, Ucan HI (2012) Aromatic chromophore-tethered Schiff base ligands and their iron (III)/chromium (III) salen and saloph capped complexes. J Fluoresc 22(5):1357–1370
Abu-Shawish HM (2009) A mercury(II) selective sensor based on N,N-bis(salicylaldehyde)-phenylenediamine as neutral carrier for potentiometric analysis in water samples. J Hazard Mater 167:602–608
Lü F, Gao L, Li H, Ding L, Fang Y (2007) Molecular engineered silica surfaces with an assembled anthracene monolayer as a fluorescent sensor for organic copper(II) salts. Appl Surf Sci 253:4123–4131
Gholivand MB, Rahimi-Nasrabadi M, Ganjali MR, Salavati-Niasari M (2007) Highly selective and sensitive copper membrane electrode based on a new synthesized Schiff base. Talanta 73:553–560
Çetindere S, Cosut B, Yeşilot S, Durmuş M, Kılıç A (2014) Synthesis and properties of axially-BODIPY conjugated subphthalocyanine dyads. Dyes Pigments 101:234–239
Khan TK, Bröring M, Mathur S, Ravikanth M (2013) Boron dipyrrin-porphyrin conjugates. Coord Chem Rev 257:2348–2387
Bayrakcı M, Kursunlu AN, Güler E, Ertul Ş (2013) A new calix[4]azacrown ether based boradiazaindacene (Bodipy): selective fluorescence changes towards trivalent lanthanide ions. Dyes Pigments 99:268–274
Kursunlu AN, Guler E, Ucan HI, Boyle RW (2012) A novel bodipy-dipyrrin fluorescent probe: synthesis and recognition behaviour towards Fe (II) and Zn (II). Dyes Pigments 94:496–502
Atilgan S, Kutuk I, Ozdemir T (2010) A near IR di-styryl BODIPY-based ratiometric fluorescent chemosensor for Hg(II). Tetrahedron Lett 51:892–894
Kursunlu AN, Deveci P, Guler E (2013) Synthesis and spectroscopic–electrochemical properties of novel ratiometric Hg (II) chemosensor containing Bodipy and the N-phenylaza-15-crown-5 moiety. J Lumin 136:430–436
Kostereli Z, Ozdemir T, Buyukcakir O, Akkaya EU (2012) Letter Tetrastyryl-BODIPY-based dendritic light harvester and estimation of energy transfer efficiency. Org Lett 14:3636–3639
Kursunlu AN, Koc ZE, Obalı AY, Güler E (2014) A symmetric and selective fluorescent Cu (II) sensor based on bodipy and s-triazine. J Lumin 149:215–220
Kursunlu AN (2015) Synthesis and photophysical properties of modifiable single, dual, and triple-boron dipyrromethene (Bodipy) complexes. Tetrahedron Lett 56:1873–1877
Findeisen-Tandel S, Weissflog W, Baumeister U, Pelzl G, Murthy HNS, Yelamaggad CV (2012) Laterally substituted symmetric and nonsymmetric salicylideneimine-based bent-core mesogens. Beilstein J Org Chem 8:129–154
Shakya PR, Singh AK, Rao TR (2012) Complexes of some 4f metal ions of the mesogenic Schiff-base, N,N9-di-(4-decyloxysalicylidene)-19,39-diaminobenzene: synthesis and spectral studies. J Coord Chem 65:3519–3529
Malkondu S, Turhan D, Kocak A (2015) Copper(II)-directed static excimer formation of an anthracene-based highly selective fluorescent receptor. Tetrahedron Lett 56:162–167
Tümay SO, Okutan E, Sengul IF, Özcan E, Kandemir H, Doruk T, Çetin M, Çosut B (2016) Naked-eye fluorescent sensor for Cu(II) based on indole conjugate BODIPY dye. Polyhedron 117:161–171
Aksuner N, Henden E, Yilmaz I, Cukurovali A (2009) A highly sensitive and selective fluorescent sensor for the determination of copper(II) based on a schiff base. Dyes Pigments 83:211–217
Li Q, Guo Y, Shao S (2012) A BODIPY based fluorescent chemosensor for Cu(II) ions and homocysteine/cysteine. Sensors Actuators B 171–172:872–877
Zeyrek CT, Elmali A, Elerman Y, Svoboda I (2005) Crystal structure and magnetic exchange interaction in a binuclear copper(II) Schiff Base complex with a bridging m-phenylenediamine ligand. Z Naturforsch 60b:143–148
Jiang X-J, Fu Y, Tang H, Zang S-Q, Hou H-W, Mak TCW, Zhang H-Y (2014) A new highly selective fluorescent sensor for detection of Cd2+ and Hg2+ based on two different approaches in aqueous solution. Sensors Actuators B 190:844–850
Acknowledgments
We thanks to Tübitak (114Z095) for financial support of this work. The authors express their appreciation to Prof. Ross W. Boyle for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kursunlu, A.N., Şahin, E. & Güler, E. Cu (II) Chemosensor Based on a Fluorogenic Bodipy-Salophen Combination: Sensitivity and Selectivity Studies. J Fluoresc 26, 1997–2004 (2016). https://doi.org/10.1007/s10895-016-1893-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1893-1