Abstract
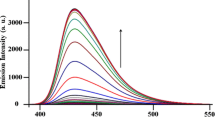
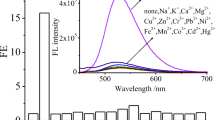
In the presented paper we investigated a 2-pyridylthiazole derivative, 4-phenyl-2-(2-pyridyl)thiazole (2-PTP), as the molecular fluorescent switches. It was firstly found that 2-PTP could perform a “turn-on” fluorescent sensing for Fe(III) with selectivity and reversibility. A 2:1 stoichiometry between 2-PTP and Fe(III) was determined according to the molar ratio method. The binding constant was evaluated as (1.90 ± 0.05) × 105 (L/mol)2. The detection limit was found as 2.2 × 10−7 M (S/N = 3). Secondly, 2-PTP also exhibited a pH-dependent dual-emission. The pK a(2-PTP-H+/2-PTP) value was then estimated as 2.0. To explain the identical emission at 479 nm of both the Fe(III) coordinated form and the protonated form of the ligand, we proposed a “locked” conformation. Finally, combining the two external stimuli as inputs, an OR logic gate was constructed using the fluorescent emission at 479 nm as the output channel.










Similar content being viewed by others
References
de Silva AP, Gunaratne HQN, Gunnaugsson T, Huxley AJM, McCoy CP, Radmacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Valuer B (2002) Molecular fluorescence: principles and applications. Wiley-VCH, Weinheim
Feringa BL (2001) Molecular switches. Wiley-VCH, Weinheim
Szacilowski K (2008) Digital information processing in molecular systems. Chem Rev 108:3481–3548
Gonçalves MST (2009) Fluorescent labeling of biomolecules with organic probes. Chem Rev 109:190–212
Terai T, Nagano T (2013) Small-molecule fluorophores and fluorescent probes for bioimaging. Pflugers Arch - Eur J Physiol 465:347–359
Dai S, Schwendtmayer C, Schürmann P, Ramaswamy S, Eklund H (2000) Redox signaling in chloroplasts: cleavage of disulfides by an iron-sulfur cluster. Science 287:655–658
Beulter E (2004) “pumping” iron: the proteins. Science 306:2051–2053
Kaplan CD, Kaplan J (2009) Iron acquisition and transcriptional regulation. Chem Rev 109:4536–4552
Theil EC, Goss DJ (2009) Living with iron (and oxygen): questions and answers about iron homeostasis. Chem Rev 109:4568–4579
Atkinson A, Winge DR (2009) Metal acquisition and availability in the mitochondria. Chem Rev 109:4708–4721
Levi S, Rovida E (2009) The role of iron in mitochondrial function. Biochim Biophys Acta 1790:629–636
Cabantchik ZI (2014) Labile iron in cells and body fluids. Physiology, pathology and pharmacology. Front Pharmacol 5: 45
Sahoo SK, Sharma D, Bera RK, Crisponi G, Callan JF (2012) Iron(III) selective molecular and supramolecular fluorescent probes. Chem Soc Rev 41:7195–7227
Wang B, Hai J, Liu Z, Wang Q, Yang Z, Sun S (2010) Selective detection of iron(III) by rhodamine-modified Fe3O4 nanoparticles. Angew Chem Int Ed 49:4576–4579
Lee MH, Giap TV, Kim SH, Lee YH, Kang C, Kim JS (2010) A novel strategy to selectively detect Fe(III) in aqueous media driven by hydrolysis of a rhodamine 6G Schiff base. Chem Commun 46:1407–1409
Yang Z, She M, Yin B, Cui J, Zhang Y, Sun W, Li J, Shi Z (2011) Three rhodamine-based “off-on” chemosensors with high selectivity and sensitivity for Fe3+ imaging in living cells. J Organomet Chem 77:1143–1147
Chen W-D, Gong W-T, Ye Z-Q, Lin Y, Ning G-L (2013) FRET-based ratiometric fluorescent probes for selective Fe3+ sensing and their applications in mitochondria. Dalton Trans 42:10093–10096
Sui B, Tang S, Liu T, Kim B, Belfield KD (2014) Novel BODIPY-based fluorescence turn-on sensor for Fe3+ and its bioimaging application in living cells. ACS Appl Mater Interfaces 6:18408–18412
Li G, Tang J, Ding P, Ye Y (2015) A rohdamine-benzimidazole based chemosensor for Fe3+ and its application in living cells. J Fluoresc. doi:10.1007/s10895-0151696-9
Zheng M-H, Jin J-Y, Sun W, Yan C-H (2006) A new series of fluorescent 5-methoxy-2-pyridylthiazoles with a pH-sensitive dual-emission. New J Chem 30:1196–1196
Zheng M-H, Zhang M-M, Li H-H, Jin J-Y (2012) Digital pH fluorescent sensing shown by small organic molecules. J Fluoresc 22:1421–1424
Zheng M-H, Sun W, Jin J-Y, Yan C-H (2014) Molecular keypad locks based on gated photochromism and enhanced fluorescence by protonation effects. J Fluoresc 14:1169–1176
Zheng M-H, Hu X, Yang M-Y, Jin J-Y (2015) Ratiometrically fluorescent sensing of Zn(II) based on dual-emission of 2-pyridylthiazole derivatives. J Fluoresc 25:1831–1834
Khoroshilov GE, Yarotskii YV, Brovarets VS, Chernega AN (2010) Synthesis and structure of N-(aroylmethyl)-2-(4-aryl-2-thiazolyl)pyridinium bromides. Zh Org Farm Khim 8:57–60
Knott RF, Breckenridge JG (1954) Analogues of 2,2′-bipyridyl with isoquinoline and thiazole rings. Part I. Can J Chem 32:512–522
Yang R, Li K, Wang K, Zhao F, Li N, Liu F (2003) Porphyrin assembly on β-cyclodextrin for selective sensing and detection of a zinc ion based on the dual emission fluorescence ratio. Anal Chem 75:612–621
Du J, Fan J, Peng X, Li H, Sun S (2010) The quinoline derivative of ratiometric and sensitive fluorescent zinc probe based on deprotonation. Sensors Actuators B 144:337–341
Shortreed M, Kopelman R, Kuhn M, Hoyland B (1997) Fluorescent fiber-optic calcium sensor for physiological measurements. Anal Chem 68:1414–1418
Acknowledgments
We thanks the financial supports from the National Natural Science Foundation of China (NSFC 21062023).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, MY., Zhao, XL., Zheng, MH. et al. Fluorescent Sensing of both Fe(III) and pH Based on 4-Phenyl-2-(2-Pyridyl)Thiazole and Construction of OR Logic Function. J Fluoresc 26, 1653–1657 (2016). https://doi.org/10.1007/s10895-016-1855-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1855-7