Abstract
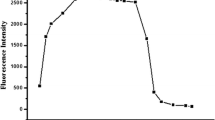
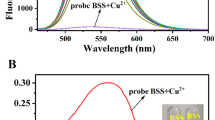
Two derivatives of fluorene containing salicylic acid groups are successfully synthesized by palladium-catalyzed coupling reactions and subsequent hydrolysis of salicylate esters. The compounds are characterized by various spectroscopic methods. In phosphate buffer (pH 8.0) solutions, these compounds are well soluble. They show maximum absorption wavelengths in the range of 304–330 nm and exhibit maximum emission wavelength around 420 and 430 nm with the quantum yields of 2.7 and 4.4 %, respectively. The compound with alkynyl salicylate groups (2) exhibits a selective fluorescence quenching towards Cu(II) and Fe(II) with a relatively similar sensitivity. The selectivity favoring Cu(II) over Fe(II) and other metal ions can be achieved upon the addition of 30 μM Triton X-100. The Cu(II) detection limit in solution phase is 1.47 ppb. The fluorescence signal recovery upon the addition of EDTA indicate a reversible complexation between 2 and Cu(II) ion. Fabrication of 2 on filter paper using a 50 μM solution in THF affords a naked-eye detection for Cu(II) and Fe(II) in aqueous media at picomole level.









Similar content being viewed by others
References
Aragay G, Pons J, Merkoci A (2011) Chem Rev 111:3433
Li X, Gao X, Shi W, Ma H (2014) Chem Rev 114:590
Zhu H, Fan J, Wang B, Peng X (2015) Chem Soc Rev 44:4337
Lakowicz JR (2006) Principles of fluorescence spectroscopy. New York, Springer
Dutta M, Das D (2012) Trends Anal Chem 32:113
Waggoner DJ, Bartnikas TB, Gitlin JD (1999) Neurobiol Dis 6:221
Strausak D, Mercer JFB, Dieter HH, Stremmel W, Multhaup G (2001) Brain Res Bull 55:175
Kumar N, Low PA (2004) J Neurol 251:747
Halfdanarson TR, Kumar N, Li C-Y, Phyliky RL, Hogan WJ (2008) Eur J Haematol 80:523
Sahin O, Akceylan E (2014) Tetrahedron 70:6944
Yeh J-T, Chen W-C, Liu S-R, Wu S-P (2014) New J Chem 35:4434
Luxami V, Gupta AS, Paul K (2014) New J Chem 35:2841
Fu Y, Feng Q-C, Jiang X-J, Xu H, Li M, Zang S-Q (2014) Dalton Trans 43:5815
Sundari R, Ahmad M, Heng LY (2006) Sensors Actuators B Chem 113:201
Sirilaksanapong S, Sukwattanasinitt M, Rashatasakhon P (2012) Chem Commun 48:293
Auttapornpitak P, Sukwattanasinitt M, Rashatasakhon P (2013) Sensors Actuators B Chem 178:296
Niamnont N, Kimpitak N, Tumcharern G, Rashatasakhon P, Sukwattanasinitt M (2013) RSC Adv 3:25215
Earmrattana N, Sukwattanasinitt M, Rashatasakhon P (2012) Dyes Pigments 93:1428
Sam-ang P, Raksasorn D, Sukwattanasinitt M, Rashatasakhon P (2014) RSC Adv 4:58077
Fracaroli AM, Furukawa H, Suzuki M, Dodd M, Okajima S, Gandara F, Reimer JA, Yaghi OM (2014) J Am Chem Soc 136:8863
Liu S, Zhang K, Lu J, Zhang J, Yip H-L, Huang F, Cao Y (2013) J Am Chem Soc 135:15326
Mallick A, Mandal MC, Haldar B, Chakrabarty A, Das P, Chattopadhyay N (2006) J Am Chem Soc 128:3126
Liana DD, Raguse B, Gooding JJ, Chow E (2012) Sensors 12:11505
Acknowledgments
This work is supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (AM1006A-56), the Ratchadapiseksomphot Endowment Fund of Chulalongkorn University (RES560530125-AM), and the Nanotechnology Center (NANOTEC), NSTDA, Ministry of Science and Technology, Thailand, through its program on Center of Excellence Network. CK thanks the 90th Anniversary of Chulalongkorn University Fund for his scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khaokeaw, C., Sukwattanasinitt, M. & Rashatasakhon, P. Salicylyl Fluorene Derivatives as Fluorescent Sensors for Cu(II) Ions. J Fluoresc 26, 745–752 (2016). https://doi.org/10.1007/s10895-016-1766-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1766-7