Abstract
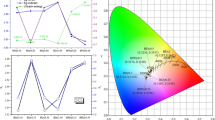
Photoluminescence studies of pure and Dy3+, Eu3+ doped Sr2CeO4 compounds are presented by oxalate precipitation method for solid state lighting. The prepared samples also characterized by XRD, SEM (EDS) and FTIR spectroscopy. The pure Sr2CeO4 compound displays a broad band in its emission spectrum when excited with 280 nm wavelength, which peaks centered at 488 nm, which is due to the energy transfer between the molecular orbital of the ligand and charge transfer state of the Ce4+ ions. Emission spectra of Sr2CeO4 with different concentration of Dy3+ ions under near UV radiation excitation, shows that intensity of luminescence spectra is found to be affected by Dy3+ ions, and it increases with adding some percentages of Dy3+ ions. The maximum doping concentration for quenching is found to be Dy3+ = 0.2 mol % to Sr2+ions. The observed broad spectrum from 400 to 560 nm is mainly due to CT transitions in Sr2CeO4 matrix and some fractional contribution of transitions between 4F9/2 → 6H15/2 of Dy3+ ions. Secondly the effect of Eu3+ doping at the Sr2+ site in Sr2CeO4, have been studied. The results obtained by doping Eu3+ concentrations (0.2 mol% to 1.5 mol%), the observed excitation and emission spectra reveal excellent energy transfer between Ce4+ and Eu3+. The phenomena of concentration quenching are explained on the basis of electron phonon coupling and multipolar interaction. This energy transfer generates white light with a color tuning from blue to red, the tuning being dependent on the Eu3+ concentration. The results establish that the compound Sr2CeO4 with Eu3+ = 1 mol% is an efficient “single host lattice” for the generation of white lights under near UV-LED and blue LED irradiation. The commission internationale de I’Eclairage (CIE) coordinates were calculated by Spectrophotometric method using the spectral energy distribution of prepared phosphors.




















Similar content being viewed by others
References
Fu S-L, Yin T, Chi F (2007) Chin Phys 16(10):3129–3133
Zambare PZ, Girase KD, Murthy KVR, Mahajan OH (2013) Adv Mat Lett 4(7):577–581
Li H et al (2014) Opt Mater 36:1883–1889
Danielson E, Devenney M, Giaquinta DM, Golden JH, Haushalter RC, McFarland EW, Poojary DM, Reaves CM, Weinberg (1998) Science 279:837–839
Seema R, Nandakumar K (2011) A New synthetic pathway of Sr2CeO4 phosphor and its characterization. J Lumin 131:2181–2184
Sukwon J, Yun Chan K, Jung Hyeun K (2007) J Mater Sci 42:9783–9794
Suresh K,Poornachandra Rao NV and Murthy KVR (2014) Bull. Mater. Sci., Vol. 37, No. 6, pp. 1191–1195, Indian Academy of Sciences
Zambare PZ, Zambare AP, Murthy KVR, Mahajan OH (2011) Ad Applied Sci Res 2(3):520–524
Walter Ratna Kumar B, Murthy KVR, Subba Rao B and Mahamuda Shaik IJSID1 (2011) (2), 145–150
Yeon woo et al. (2015) Ceramic International 41 1249–1254
Xue S-W, Wang E-G, Zhang JC (2011) Phys B 20(7):078105–078108
Masalove AA, Vyagin OG, Ganina II, Malyukin YV (2008) Funct Mater 15(4):470–474
Zhang C, Shi J, Yang X, Lu L, Wang X (2010) J Rare Earths 28(4):513–518
Takyuki H, Yusuke K (2004) J Phys Chem B 108(34):12763–12769
Ye S, Xiao F, Pan YX, Ma YY, Zhang QY (2010) Mater Sci Eng R 71:1–34
San Jose (2013) CA 95134 USA,LED Engine, 651 River Oaks Parkway, sales@ledengine.com
Na Z, Wang D, Lan L, Yanshuang M, Xiaosang Z, Ming N (2006) J Rare Earth 24:294–297
Xu Y, Chen L, Li Y, Song G, Wang Y, Zhuang W, Long Z (2008) Appl Phys Lett 92:021129
Yan W, Yuhua W, Feng Z, Bitao L (2011) Mater Chem Phys 129:1171–1175
Pawade VB, Dhobale NS, Dhobale SJ (2012) Solid State Sci 14:607–610
Pawade VB and Dhobale SJ (2011) The J. of biological and Chemical Luminescence
Bizari G, and Moine B (2006) Optical Material, 587–591
Zhu H, Yang H, Fu W, Zhu P, Li M, Li Y, Sui Y, Liu S, Zou G (2008) Mater Lett 62:784–786
Matolin V, Matolinova I, Sadlacek L, Prince KC, Skala T (2009) Nanotechnology 20:1–7
Ferrar JL, Pires AM, Serra OA, Davolos MR (2011) J Lumin 131:25–29
Sankara R, Subba Rao GV (2011) J Electrochem Soc 158(10):J287–J290
Suresh K et al (2013) J Lumin 133:96–101
Lili S, Hongiie Z, Li C, Qiang S (2011) RSC Adv 1:298–304
Haiyan JIAO, Yuhua WANG, Jiachi ZHANG (2009) J Phys Conf Ser 152:012089
Shinoya M and Yen W M (1999) Phosphor HandBook CRC Press Boca Raton
Choi S-H, Kim N-H, Yang- Hoon Y, Sung-Churl C (2006) J Cer Proc Res 7(1):62–65
Jiang YD, Zhang F, Summers CJ (1999) Appl Phys Lett 74:1677
Xing D, Gong ML, Qui X, Yang D, Cheah Kok W (2006) J Rare Earth 24:289–293
Shriver DF, Akins PW, Langford CH (1990) Inorganic chemistry, WH. Freeman and company, New York, NY
Jie L, Xi L, Hu S, Yingchun L, Yuying H (2013) Opt Mater 35:2309–2313
Aitasalo T, Holsa J, Lastusaari M, Niityykoski J, Pelle F (2005) Optic Mater 27:1511
Paulose PI, Jose G, Thomas V et al (2003) J Phys Chem Solids 64:841
Yang W, Chen T (2007) Appl Phys Lett 90:171908
Kim Anha T, Strek W (1988) J Lumin 42:205
Kim JS, Jeon PE, Park YH, Choi JC, Park HL (2004) Appl Phys Lett 85(17):3696
Fraser J. Douglas,a Carlos Renero-Lecuna, Robert D. Peacock, Rafael Valiente, Donald A. MacLarenc and Mark Murriea (2012) Electronic Supplementary Material (ESI) for CrystEngComm, the Royal Society of Chemistry
Haifeng L et al (2015) Mater Lett 139:258–261
Som S, Kunti AK, Vinod K, Vijay K, Dutta S, Chowdhury M, Sharma SK, Terblans JJ, Swart HC (2014) J Appl Phys 115:193101
Huang K-W, Chen W-T, Chu C-I, Hu S-F, Sheu H-S, Cheng B-M, Chen J-M, Liu R-S (2012) Chem Mater 24:2220
Dalton Trans (2014) 43, 8814
RSC Advances (2012) 2 10859–10868
Condon SU, Shortley GH (1963) The theory of atomic spectra. Cambridge University Press, England
Dutta S, Som S, Sharma SK (2013) Dalton Trans 42:9654
Dexter DL (1953) J Chem Phys 21:836
Dubey V, Kaur J, Agrawal S (2014) Res. Chem. Intermed. 10.1007/s11164-014-1563-3
Fang Y-C, Chu S-Y, Kao P-C, Chuang Y-M, Zeng Z-L (2011) J Electrochem Soc 158:J1
Hehlen MP, Brik MG, Kramer KW (2013) J Lumin 136:221–239
Agrawal S, Dubey V (2014) Jour Rad Res Appl Sci 7(4):601–606
Dubey V, Kaur J, Agrawal S (2014) Res Chem Intermed. doi:10.1007/s11164-014-1563-3
Dubey V, Kaur J, Agrawal S, Suryanarayana NS, Murthy KVR (2014) Superlat Microstruc 67:156–171
Kaur J, Parganiha Y, Dubey V, Singh D, Chandrakar D (2014) Superlat Microstruc 73:38–53
Dubey V, Kaur J, Agrawal S, Suryanarayana NS, Murthy KVR (2013) Optik – Int. J.Light Electron Opt. doi:10.1016/j.ijleo.2013.03.153
Parganiha Y, Kaur J, Dubey V, Murthy KVR (2015) Mater Sci Semicond Process 31:715–719
Acknowledgments
One of the authors R. S. Ukare is very much thankful to University Grant Commission, Pune, for providing financial grant under Faculty Development Programme (FDP).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ukare, R.S., Dubey, V., Zade, G.D. et al. PL Properties of Sr2CeO4 With Eu3+ and Dy3+ for Solid State Lighting Prepared by Precipitation Method. J Fluoresc 26, 791–806 (2016). https://doi.org/10.1007/s10895-016-1765-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1765-8